Nanovehicular intracellular delivery systems
- PMID: 18200527
- PMCID: PMC3747665
- DOI: 10.1002/jps.21270
Nanovehicular intracellular delivery systems
Abstract
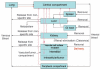
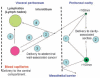
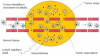
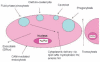
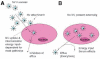
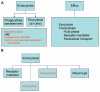
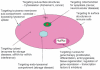
This article provides an overview of principles and barriers relevant to intracellular drug and gene transport, accumulation and retention (collectively called as drug delivery) by means of nanovehicles (NV). The aim is to deliver a cargo to a particular intracellular site, if possible, to exert a local action. Some of the principles discussed in this article apply to noncolloidal drugs that are not permeable to the plasma membrane or to the blood-brain barrier. NV are defined as a wide range of nanosized particles leading to colloidal objects which are capable of entering cells and tissues and delivering a cargo intracelullarly. Different localization and targeting means are discussed. Limited discussion on pharmacokinetics and pharmacodynamics is also presented. NVs are contrasted to micro-delivery and current nanotechnologies which are already in commercial use. Newer developments in NV technologies are outlined and future applications are stressed. We also briefly review the existing modeling tools and approaches to quantitatively describe the behavior of targeted NV within the vascular and tumor compartments, an area of particular importance. While we list "elementary" phenomena related to different level of complexity of delivery to cancer, we also stress importance of multi-scale modeling and bottom-up systems biology approach.
Figures



References
-
- Kostarelos K. Rational design and engineering of delivery systems for therapeutics: Biomedical exercises in colloid and surface science. Adv Colloid Interface Sci. 2003;106:147–168. - PubMed
-
- Moses MA, Brem H, Langer R. Advancing the field of drug delivery: Taking aim at cancer. Cancer Cell. 2003;4:337–341. - PubMed
-
- Shargel L, Yu AB. Applied biopharmaceutics & pharmacokinetics. 4th edition McGraw-Hill; New York: 1999.
-
- van de Waterbeemd H, Gifford E. ADMET in silico modelling: Towards prediction paradise? Nat Rev Drug Discov. 2003;2:192–204. - PubMed
-
- Okada H, Toguchi H. Biodegradable microspheres in drug delivery. Crit Rev Ther Drug Carrier Syst. 1995;12:1–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

