Higher persistence with valsartan compared with enalapril in daily practice
- PMID: 18200822
- PMCID: PMC2350122
Higher persistence with valsartan compared with enalapril in daily practice
Abstract
Objective: To compare persistence with valsartan and enalapril in daily practice.
Methods: The PHARMO Record Linkage System includes various data registries including drug dispensing and hospitalizations for > or =2 million subjects in the Netherlands. Patients newly treated with valsartan or enalapril in the period of 1999-2002 were selected. Persistence was calculated by summing up the number of days of continuous treatment. Patients who remained on therapy with valsartan or enalapril for 12 or 24 months were defined as persistent at 1 or 2 years, respectively.
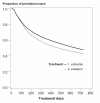
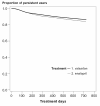
Results: 3364 patients received valsartan and 9103 patients received enalapril. About 62% of patients treated with valsartan and 55% of patients treated with enalapril remained on therapy at 12 months after the initial dispensing, while 48% of patients treated with valsartan and 43% of patients treated with enalapril were persistent at 24 months. Patients treated with valsartan were about 20% more likely to stay on treatment than patients treated with enalapril (1 year RR(adj): 1.23, 95% CI: 1.16-1.32; 2 years RR(adj): 1.16, 95% CI: 1.11-1.23).
Conclusions: Real-life persistence is higher with valsartan than with enalapril. The results of this and other studies on persistence in daily practice should be taken into account when deciding upon drug treatment for hypertension.
Figures
References
-
- Bourgault C, Senecal M, Brisson M, et al. Persistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population-based study. J Hum Hypertens. 2005;19:607–13. - PubMed
-
- Breekveldt-Postma NS, Herings RM. Persistence with antihypertensives related to formulation: the case of nifedipine. Ann Pharmacother. 2005;39:237–42. - PubMed
-
- Catalan V, Lelorier J. Predictors of long-term persistence on statins in a subsidized clinical population. Value in Health. 2000;3:417–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

