Cytomegalovirus inhibition of embryonic mouse tooth development: a model of the human amelogenesis imperfecta phenocopy
- PMID: 18201685
- PMCID: PMC2279100
- DOI: 10.1016/j.archoralbio.2007.11.014
Cytomegalovirus inhibition of embryonic mouse tooth development: a model of the human amelogenesis imperfecta phenocopy
Abstract
Objective: Cytomegalovirus (CMV) is one of the most common causes of major birth defects in humans. Of the approximately 8400 children born each year in the U.S. with CMV-induced birth defects, more than 1/3 of these children exhibit hypoplasia and hypocalcification of tooth enamel. Our objective was to initiate the investigation of the pathogenesis of CMV-induced tooth defects.
Design: Mouse Cap stage mandibular first molars were infected with mouse CMV (mCMV) in vitro in a chemically-defined organ culture system and analysed utilising histological and immunolocalisation methodologies. The antiviral, acyclovir, was used to inhibit mCMV replication and comparisons made between mCMV-infected and acyclovir-treated, mCMV-infected teeth.
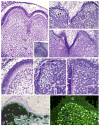
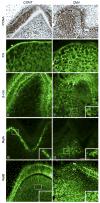
Results: Active infection of Cap stage molars for up to 15 days in vitro results in smaller, developmentally-delayed and dysmorphic molars characterised by shallow, broad and misshapen cusps, infected and affected dental papilla mesenchyme, poorly differentiated odontoblasts and ameloblasts, and no dentin matrix. Initial protein localisation studies suggest that the pathogenesis is mediated through NF-kappaB signaling and that there appears to be an unusual interaction between abnormal mesenchymal cells and surrounding matrix. Rescue with acyclovir indicates that mCMV replication is necessary to initiate and sustain progressive tooth dysmorphogenesis.
Conclusions: Our results indicate that mCMV-induced changes in signaling pathways severely delays, but does not completely interrupt, tooth morphogenesis. Importantly, our results demonstrate that this well-defined embryonic mouse organ culture system can be utilised to delineate the molecular mechanism underlying the CMV-induced tooth defects that characterise the amelogenesis imperfecta phenocopy seen in many CMV-infected children.
Figures

References
-
- Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al., editors. Fields virology. 4th. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2675–705.
-
- Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355–63. - PubMed
-
- Ross DS, Dollard SC, Victor M, Sumartojo E, Cannon MJ. The epidemiology and prevention of congenital cytomegalovirus infection and disease: activities of the Centers for Disease Control and Prevention Workgroup. J Womens Health (Larchmt) 2006;15:224–9. - PubMed
-
- Stagno S, Pass RF, Dworsky ME, Alford CA. Congenital and perinatal cytomegalovirus infections. Semin Perinatol. 1983;7:31–42. - PubMed
-
- Stagno S, Pass RF, Dworsky ME, Britt WJ, Alford CA. Congenital and perinatal cytomegalovirus infections: clinical characteristics and pathogenic factors. Birth Defects Orig Article Ser. 1984;20:65–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

