Enabled (Xena) regulates neural plate morphogenesis, apical constriction, and cellular adhesion required for neural tube closure in Xenopus
- PMID: 18201691
- PMCID: PMC2266839
- DOI: 10.1016/j.ydbio.2007.12.010
Enabled (Xena) regulates neural plate morphogenesis, apical constriction, and cellular adhesion required for neural tube closure in Xenopus
Abstract
Regulation of cellular adhesion and cytoskeletal dynamics is essential for neurulation, though it remains unclear how these two processes are coordinated. Members of the Ena/VASP family of proteins are localized to sites of cellular adhesion and actin dynamics and lack of two family members, Mena and VASP, in mice results in failure of neural tube closure. The precise mechanism by which Ena/VASP proteins regulate this process, however, is not understood. In this report, we show that Xenopus Ena (Xena) is localized to apical adhesive junctions of neuroepithelial cells during neurulation and that Xena knockdown disrupts cell behaviors integral to neural tube closure. Changes in the shape of the neural plate as well as apical constriction within the neural plate are perturbed in Xena knockdown embryos. Additionally, we demonstrate that Xena is essential for cell-cell adhesion. These results demonstrate that Xena plays an integral role in coordinating the regulation of cytoskeletal dynamics and cellular adhesion during neurulation in Xenopus.
Figures
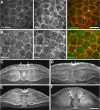


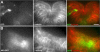


Similar articles
-
Cloning and developmental expression of Xenopus Enabled (Xena).Dev Dyn. 2005 Jun;233(2):631-7. doi: 10.1002/dvdy.20358. Dev Dyn. 2005. PMID: 15778995
-
Formin homology 2 domain-containing 3 (Fhod3) controls neural plate morphogenesis in mouse cranial neurulation by regulating multidirectional apical constriction.J Biol Chem. 2019 Feb 22;294(8):2924-2934. doi: 10.1074/jbc.RA118.005471. Epub 2018 Dec 20. J Biol Chem. 2019. PMID: 30573686 Free PMC article.
-
Regulation of somitogenesis by Ena/VASP proteins and FAK during Xenopus development.Development. 2006 Feb;133(4):685-95. doi: 10.1242/dev.02230. Epub 2006 Jan 18. Development. 2006. PMID: 16421193
-
TRPM6 and TRPM7: Novel players in cell intercalation during vertebrate embryonic development.Dev Dyn. 2020 Aug;249(8):912-923. doi: 10.1002/dvdy.182. Epub 2020 May 26. Dev Dyn. 2020. PMID: 32315468 Review.
-
Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure.Dev Growth Differ. 2012 Apr;54(3):266-76. doi: 10.1111/j.1440-169X.2012.01346.x. Dev Growth Differ. 2012. PMID: 22524600 Review.
Cited by
-
A novel genetic mechanism regulates dorsolateral hinge-point formation during zebrafish cranial neurulation.J Cell Sci. 2009 Jun 15;122(Pt 12):2137-48. doi: 10.1242/jcs.043471. Epub 2009 May 26. J Cell Sci. 2009. PMID: 19470582 Free PMC article.
-
Totally tubular: the mystery behind function and origin of the brain ventricular system.Bioessays. 2009 Apr;31(4):446-58. doi: 10.1002/bies.200800207. Bioessays. 2009. PMID: 19274662 Free PMC article. Review.
-
The continuing challenge of understanding, preventing, and treating neural tube defects.Science. 2013 Mar 1;339(6123):1222002. doi: 10.1126/science.1222002. Science. 2013. PMID: 23449594 Free PMC article. Review.
-
Vinculin is required for interkinetic nuclear migration (INM) and cell cycle progression.J Cell Biol. 2024 Jan 1;223(1):e202106169. doi: 10.1083/jcb.202106169. Epub 2023 Oct 27. J Cell Biol. 2024. PMID: 37889294 Free PMC article.
-
The lens actin filament cytoskeleton: Diverse structures for complex functions.Exp Eye Res. 2017 Mar;156:58-71. doi: 10.1016/j.exer.2016.03.005. Epub 2016 Mar 10. Exp Eye Res. 2017. PMID: 26971460 Free PMC article. Review.
References
-
- Aberle H, et al. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23. - PubMed
-
- Bear JE, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–21. - PubMed
-
- Bertet C, et al. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. - PubMed
-
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

