Connective tissue growth factor/CCN2-null mouse embryonic fibroblasts retain intact transforming growth factor-beta responsiveness
- PMID: 18201696
- PMCID: PMC3963386
- DOI: 10.1016/j.yexcr.2007.12.010
Connective tissue growth factor/CCN2-null mouse embryonic fibroblasts retain intact transforming growth factor-beta responsiveness
Abstract
Background: The matricellular protein connective tissue growth factor (CCN2) has been implicated in pathological fibrosis, but its physiologic role remains elusive. In vitro, transforming growth factor-beta (TGF-beta) induces CCN2 expression in mesenchymal cells. Because CCN2 can enhance profibrotic responses elicited by TGF-beta, it has been proposed that CCN2 functions as an essential downstream signaling mediator for TGF-beta. To explore this notion, we characterized TGF-beta-induced activation of fibroblasts from CCN2-null (CCN2(-/-)) mouse embryos.
Methods: The regulation of CCN2 expression was examined in vivo in a model of fibrosis induced by bleomycin. Cellular TGF-beta signal transduction and regulation of collagen gene expression were examined in CCN2(-/-) MEFs by immunohistochemistry, Northern, Western and RT-PCR analysis, immunocytochemistry and transient transfection assays.
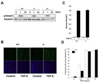
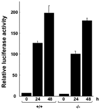
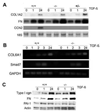
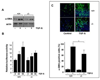
Results: Bleomycin-induced skin fibrosis in the mouse was associated with substantial CCN2 up-regulation in lesional fibroblasts. Whereas in vitro proliferation rate of CCN2(-/-) MEFs was markedly reduced compared to wild type MEFs, TGF-beta-induced activation of the Smad pathways, including Smad2 phosphorylation, Smad2/3 and Smad4 nuclear accumulation and Smad-dependent transcriptional responses, were unaffected by loss of CCN2. The stimulation of COL1A2 and fibronectin mRNA expression and promoter activity, and of corresponding protein levels, showed comparable time and dose-response in wild type and CCN2(-/-) MEFs, whereas stimulation of alpha smooth muscle actin and myofibroblast transdifferentiation showed subtle impairment in MEFs lacking CCN2.
Conclusion: Whereas endogenous CCN2 plays a role in regulation of proliferation and TGF-beta-induced myofibroblast transdifferentiation, it appears to be dispensable for Smad-dependent stimulation of collagen and extracellular matrix synthesis in murine embryonic fibroblasts.
Figures





References
-
- Jimenez SA, Derk CT. Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis. Ann Intern Med. 2004 Jan 6;140(1):37–50. - PubMed
-
- Massague J, Gomis RR. The logic of TGF-beta signaling. FEBS Lett. 2006 May 22;580(12):2811–2820. - PubMed
-
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005 Aug 15;118:3573–3584. - PubMed
-
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004 Jan 3;363(9402):62–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

