Activated polymorphonuclear cells promote injury and excitability of dorsal root ganglia neurons
- PMID: 18201702
- PMCID: PMC2397441
- DOI: 10.1016/j.expneurol.2007.11.024
Activated polymorphonuclear cells promote injury and excitability of dorsal root ganglia neurons
Abstract
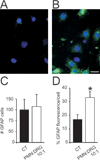
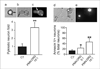
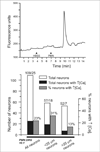
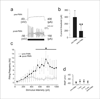
Therapies aimed at depleting or blocking the migration of polymorphonuclear leukocytes (PMN or neutrophils) are partially successful in the treatment of neuroinflammatory conditions and in attenuating pain following peripheral nerve injury or subcutaneous inflammation. However, the functional effects of PMN on peripheral sensory neurons such as dorsal root ganglia (DRG) neurons are largely unknown. We hypothesized that PMN are detrimental to neuronal viability in culture and increase neuronal activity and excitability. We demonstrate that isolated peripheral PMN are initially in a relatively resting state but undergo internal oxidative burst and activation by an unknown mechanism within 10 min of co-culture with dissociated DRG cells. Co-culture for 24 h decreases neuronal count at a threshold<0.4:1 PMN:DRG cell ratio and increases the number of injured and apoptotic neurons. Within 3 min of PMN addition, fluorometric calcium imaging reveals intracellular calcium transients in small size (<25 microm diam) and large size (>25 microm diam) neurons, as well as in capsaicin-sensitive neurons. Furthermore, small size isolectin B4-labeled neurons undergo hyperexcitability manifested as decreased current threshold and increased firing frequency. Although co-culture of PMN and DRG cells does not perfectly model neuroinflammatory conditions in vivo, these findings suggest that activated PMN can potentially aggravate neuronal injury and cause functional changes to peripheral sensory neurons. Distinguishing the beneficial from the detrimental effects of PMN on neurons may aid in the development of more effective drug therapies for neurological disorders involving neuroinflammation, including painful neuropathies.
Figures

Similar articles
-
An improved method for patch clamp recording and calcium imaging of neurons in the intact dorsal root ganglion in rats.J Neurosci Methods. 2008 Aug 15;173(1):74-82. doi: 10.1016/j.jneumeth.2008.05.023. Epub 2008 Jun 7. J Neurosci Methods. 2008. PMID: 18588915 Free PMC article.
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
A-type voltage-gated K+ currents influence firing properties of isolectin B4-positive but not isolectin B4-negative primary sensory neurons.J Neurophysiol. 2005 Jun;93(6):3401-9. doi: 10.1152/jn.01267.2004. Epub 2005 Jan 12. J Neurophysiol. 2005. PMID: 15647393
-
Local inflammation in rat dorsal root ganglion alters excitability and ion currents in small-diameter sensory neurons.Anesthesiology. 2007 Aug;107(2):322-32. doi: 10.1097/01.anes.0000270761.99469.a7. Anesthesiology. 2007. PMID: 17667578 Free PMC article.
-
Functional expression of P2X7 receptors in non-neuronal cells of rat dorsal root ganglia.Brain Res. 2005 Aug 2;1052(1):63-70. doi: 10.1016/j.brainres.2005.06.022. Brain Res. 2005. PMID: 16005856
Cited by
-
DNA damage response in peripheral nervous system: coping with cancer therapy-induced DNA lesions.DNA Repair (Amst). 2013 Aug;12(8):685-90. doi: 10.1016/j.dnarep.2013.04.020. Epub 2013 May 16. DNA Repair (Amst). 2013. PMID: 23684797 Free PMC article. Review.
-
Inflammation and the neurovascular unit in the setting of focal cerebral ischemia.Neuroscience. 2009 Feb 6;158(3):972-82. doi: 10.1016/j.neuroscience.2008.08.028. Epub 2008 Aug 27. Neuroscience. 2009. PMID: 18824084 Free PMC article. Review.
-
A potential therapeutic target: The role of neutrophils in the central nervous system.Brain Behav Immun Health. 2023 Sep 17;33:100688. doi: 10.1016/j.bbih.2023.100688. eCollection 2023 Nov. Brain Behav Immun Health. 2023. PMID: 37767236 Free PMC article. Review.
-
Characterization of neutrophil-neuronal co-cultures to investigate mechanisms of post-ischemic immune-mediated neurotoxicity.J Neurosci Methods. 2020 Jul 15;341:108782. doi: 10.1016/j.jneumeth.2020.108782. Epub 2020 May 20. J Neurosci Methods. 2020. PMID: 32445795 Free PMC article.
-
Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA.J Immunol. 2012 Jul 1;189(1):381-92. doi: 10.4049/jimmunol.1200409. Epub 2012 Jun 1. J Immunol. 2012. PMID: 22661091 Free PMC article.
References
-
- Bailey AL, Ribeiro-da-Silva A. Transient loss of terminals from non-peptidergic nociceptive fibers in the substantia gelatinosa of spinal cord following chronic constriction injury of the sciatic nerve. Neuroscience. 2006;138:675–690. - PubMed
-
- Bennett G, al-Rashed S, Hoult JR, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998;77:315–322. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

