N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility
- PMID: 18201716
- PMCID: PMC2314673
- DOI: 10.1016/j.yjmcc.2007.11.013
N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility
Abstract
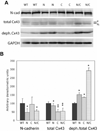
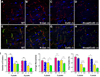
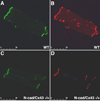
Cardiac-specific deletion of the murine gene (Cdh2) encoding the cell adhesion molecule, N-cadherin, results in disassembly of the intercalated disc (ICD) structure and sudden arrhythmic death. Connexin 43 (Cx43)-containing gap junctions are significantly reduced in the heart after depleting N-cadherin, therefore we hypothesized that animals expressing half the normal levels of N-cadherin would exhibit an intermediate phenotype. We examined the effect of N-cadherin haploinsufficiency on Cx43 expression and susceptibility to induced arrhythmias in mice either wild-type or heterozygous for the Cx43 (Gja1)-null allele. An increase in hypophosphorylated Cx43 accompanied by a modest decrease in total Cx43 protein levels was observed in the N-cadherin heterozygous mice. Consistent with these findings N-cadherin heterozygotes exhibited increased susceptibility to ventricular arrhythmias compared to wild-type mice. Quantitative immunofluorescence microscopy revealed a reduction in size of large Cx43-containing plaques in the N-cadherin heterozygous animals compared to wild-type. Gap junctions were further decreased in number and size in the N-cad/Cx43 compound heterozygous mice with increased arrhythmic susceptibility compared to the single mutants. The scaffold protein, ZO-1, was reduced at the ICD in N-cadherin heterozygous cardiomyocytes providing a possible explanation for the reduction in Cx43 plaque size. These data provide further support for the intimate relationship between N-cadherin and Cx43 in the heart, and suggest that germline mutations in the human N-cadherin (Cdh2) gene may predispose patients to increased risk of cardiac arrhythmias.
Figures




Similar articles
-
Loss of cadherin-binding proteins β-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis.Mol Cell Biol. 2012 Mar;32(6):1056-67. doi: 10.1128/MCB.06188-11. Epub 2012 Jan 17. Mol Cell Biol. 2012. PMID: 22252313 Free PMC article.
-
Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions.Circ Res. 2002 Feb 22;90(3):317-24. doi: 10.1161/hh0302.104471. Circ Res. 2002. PMID: 11861421
-
Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes.J Cell Sci. 2014 Mar 1;127(Pt 5):1104-16. doi: 10.1242/jcs.143743. Epub 2014 Jan 10. J Cell Sci. 2014. PMID: 24413171 Free PMC article.
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
-
Electrical consequences of cardiac myocyte: fibroblast coupling.Biochem Soc Trans. 2015 Jun;43(3):513-8. doi: 10.1042/BST20150035. Biochem Soc Trans. 2015. PMID: 26009200 Review.
Cited by
-
Essential role of obscurin kinase-1 in cardiomyocyte coupling via N-cadherin phosphorylation.JCI Insight. 2024 Feb 8;9(3):e162178. doi: 10.1172/jci.insight.162178. JCI Insight. 2024. PMID: 38127465 Free PMC article.
-
Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection.Pharmacol Ther. 2015 Sep;153:90-106. doi: 10.1016/j.pharmthera.2015.06.005. Epub 2015 Jun 11. Pharmacol Ther. 2015. PMID: 26073311 Free PMC article. Review.
-
Effects of ischemic preconditioning on ischemia/reperfusion-induced arrhythmias by upregulatation of connexin 43 expression.J Cardiothorac Surg. 2011 Jun 2;6:80. doi: 10.1186/1749-8090-6-80. J Cardiothorac Surg. 2011. PMID: 21635761 Free PMC article.
-
Alterations in cell adhesion proteins and cardiomyopathy.World J Cardiol. 2014 May 26;6(5):304-13. doi: 10.4330/wjc.v6.i5.304. World J Cardiol. 2014. PMID: 24944760 Free PMC article. Review.
-
Xin Scaffolding Proteins and Arrhythmias.J Cardiol Clin Res. 2013 Oct 24;1(2):1011. J Cardiol Clin Res. 2013. PMID: 24734257 Free PMC article. No abstract available.
References
-
- Akar FG, Tomaselli GF. Conduction abnormalities in nonischemic dilated cardiomyopathy: basic mechanisms and arrhythmic consequences. Trends Cardiovasc Med. 2005;15(7):259–264. - PubMed
-
- Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10(4):169–177. - PubMed
-
- Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97(17):1746–1754. - PubMed
-
- van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109(8):1048–1055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

