Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib
- PMID: 18202011
- PMCID: PMC2390754
- DOI: 10.1158/1535-7163.MCT-07-0584
Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib
Abstract
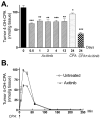
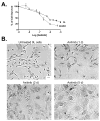
The promising but still limited efficacy of angiogenesis inhibitors as monotherapies for cancer treatment indicates a need to integrate these agents into existing therapeutic regimens. Presently, we investigate the antitumor activity of the small-molecule angiogenesis inhibitor axitinib (AG-013736) and its potential for combination with metronomic cyclophosphamide. Axitinib significantly inhibited angiogenesis in rat 9L tumors grown s.c. in scid mice but only moderately delayed tumor growth. Combination of axitinib with metronomic cyclophosphamide fully blocked 9L tumor growth on initiation of drug treatment. In contrast, metronomic cyclophosphamide alone required multiple treatment cycles to halt tumor growth. However, in contrast to the substantial tumor regression that is ultimately induced by metronomic cyclophosphamide, the axitinib/cyclophosphamide combination was tumor growth static. Axitinib did not inhibit hepatic activation of cyclophosphamide or export of its activated metabolite, 4-hydroxy-cyclophosphamide (4-OH-CPA), to extrahepatic tissues; rather, axitinib selectively decreased 9L tumor uptake of 4-OH-CPA by 30% to 40%. The reduced tumor penetration of 4-OH-CPA was associated with a decrease in cyclophosphamide-induced tumor cell apoptosis and a block in the induction of the endogenous angiogenesis inhibitor thrombospondin-1 in tumor-associated host cells, which may contribute to the absence of tumor regression with the axitinib/cyclophosphamide combination. Finally, axitinib transiently increased 9L tumor cell apoptosis, indicating that its effects are not limited to the endothelial cell population. These findings highlight the multiple effects that may characterize antiangiogenic agent/metronomic chemotherapy combinations and suggest that careful optimization of drug scheduling and dosages will be required to maximize antitumor responses.
Figures






Similar articles
-
Dominant effect of antiangiogenesis in combination therapy involving cyclophosphamide and axitinib.Clin Cancer Res. 2009 Jan 15;15(2):578-88. doi: 10.1158/1078-0432.CCR-08-1174. Clin Cancer Res. 2009. PMID: 19147763 Free PMC article.
-
Collaboration between hepatic and intratumoral prodrug activation in a P450 prodrug-activation gene therapy model for cancer treatment.Mol Cancer Ther. 2007 Nov;6(11):2879-90. doi: 10.1158/1535-7163.MCT-07-0297. Epub 2007 Nov 7. Mol Cancer Ther. 2007. PMID: 17989319 Free PMC article.
-
Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer.Mol Cancer Ther. 2010 Apr;9(4):996-1006. doi: 10.1158/1535-7163.MCT-09-0960. Epub 2010 Apr 6. Mol Cancer Ther. 2010. PMID: 20371722 Free PMC article.
-
Upregulation of endogenous angiogenesis inhibitors: a mechanism of action of metronomic chemotherapy.Cancer Biol Ther. 2004 Dec;3(12):1212-3. doi: 10.4161/cbt.3.12.1369. Epub 2004 Dec 15. Cancer Biol Ther. 2004. PMID: 15662130 Review.
-
Does axitinib (AG-01376) have a future role in metastatic renal cell carcinoma and other malignancies?Expert Rev Anticancer Ther. 2010 Oct;10(10):1545-57. doi: 10.1586/era.10.134. Expert Rev Anticancer Ther. 2010. PMID: 20942625 Review.
Cited by
-
Anti-angiogenic treatments in advanced NSCLC: back to the drawing board.J Thorac Dis. 2012 Dec;4(6):643-6. doi: 10.3978/j.issn.2072-1439.2012.10.11. J Thorac Dis. 2012. PMID: 23205293 Free PMC article. No abstract available.
-
Targeting drug-metabolizing enzymes for effective chemoprevention and chemotherapy.Drug Metab Dispos. 2010 Apr;38(4):539-44. doi: 10.1124/dmd.109.031351. Drug Metab Dispos. 2010. PMID: 20233842 Free PMC article.
-
Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor.Clin Cancer Res. 2011 Sep 1;17(17):5656-67. doi: 10.1158/1078-0432.CCR-11-0078. Epub 2011 Jul 25. Clin Cancer Res. 2011. PMID: 21788355 Free PMC article.
-
Which drug or drug delivery system can change clinical practice for brain tumor therapy?Neuro Oncol. 2013 Jun;15(6):656-69. doi: 10.1093/neuonc/not016. Epub 2013 Mar 15. Neuro Oncol. 2013. PMID: 23502426 Free PMC article. Review.
-
Differential Effects of Ang-2/VEGF-A Inhibiting Antibodies in Combination with Radio- or Chemotherapy in Glioma.Cancers (Basel). 2019 Mar 6;11(3):314. doi: 10.3390/cancers11030314. Cancers (Basel). 2019. PMID: 30845704 Free PMC article.
References
-
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. - PubMed
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–74. - PubMed
-
- Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33(5 Suppl 10):S1–7. - PubMed
-
- Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312(5777):1171–5. - PubMed
-
- Nieder C, Wiedenmann N, Andratschke N, Molls M. Current status of angiogenesis inhibitors combined with radiation therapy. Cancer Treat Rev. 2006;32(5):348–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

