Effects of Muclin (Dmbt1) deficiency on the gastrointestinal system
- PMID: 18202109
- PMCID: PMC3760339
- DOI: 10.1152/ajpgi.00525.2007
Effects of Muclin (Dmbt1) deficiency on the gastrointestinal system
Abstract
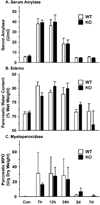
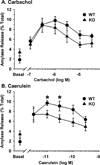
The Dmbt1 gene encodes alternatively spliced glycoproteins that are either membrane-associated or secreted epithelial products. Functions proposed for Dmbt1 include it being a tumor suppressor, having roles in innate immune defense and inflammation, and being a Golgi-sorting receptor in the exocrine pancreas. The heavily sulfated membrane glycoprotein mucin-like glycoprotein (Muclin) is a Dmbt1 product that is strongly expressed in organs of the gastrointestinal (GI) system. To explore Muclin's functions in the GI system, the Dmbt1 gene was targeted to produce Muclin-deficient mice. Muclin-deficient mice have normal body weight gain and are fertile. The Muclin-deficient mice did not develop GI tumors, even when crossed with mice lacking the known tumor suppressor p53. When colitis was induced by dextran sulfate sodium, there was no significant difference in disease severity in Muclin-deficient mice. Also, when acute pancreatitis was induced with supraphysiological caerulein, there was no difference in disease severity in the Muclin-deficient mice. Exocrine pancreatic function was impaired, as measured by attenuated neurohormonal-stimulated amylase release from Muclin-deficient acinar cells. Also, by [(35)S]Met/Cys pulse-chase analysis, traffic of newly synthesized protein to the stimulus-releasable pool was significantly retarded in Muclin-deficient cells compared with wild type. Thus Muclin deficiency impairs trafficking of regulated proteins to a stimulus-releasable pool in the exocrine pancreas.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures




Similar articles
-
Binding of the Golgi sorting receptor muclin to pancreatic zymogens through sulfated O-linked oligosaccharides.J Biol Chem. 2004 Sep 24;279(39):40918-26. doi: 10.1074/jbc.M406213200. Epub 2004 Jul 29. J Biol Chem. 2004. PMID: 15292166
-
Processing of pro-Muclin and divergent trafficking of its products to zymogen granules and the apical plasma membrane of pancreatic acinar cells.Eur J Cell Biol. 2000 Dec;79(12):892-904. doi: 10.1078/0171-9335-00121. Eur J Cell Biol. 2000. PMID: 11152281
-
Altered posttranslational processing of glycoproteins in cerulein-induced pancreatitis.Exp Cell Res. 2005 Aug 1;308(1):101-13. doi: 10.1016/j.yexcr.2005.04.003. Exp Cell Res. 2005. PMID: 15869754
-
MUCLIN expression in the cystic fibrosis transmembrane conductance regulator knockout mouse.Gastroenterology. 1997 Aug;113(2):521-32. doi: 10.1053/gast.1997.v113.pm9247472. Gastroenterology. 1997. PMID: 9247472
-
Expression of pro-Muclin in pancreatic AR42J cells induces functional regulated secretory granules.Am J Physiol Cell Physiol. 2005 Nov;289(5):C1169-78. doi: 10.1152/ajpcell.00099.2005. Epub 2005 Jun 29. Am J Physiol Cell Physiol. 2005. PMID: 15987769
Cited by
-
Replacement of Rbpj with Rbpjl in the PTF1 complex controls the final maturation of pancreatic acinar cells.Gastroenterology. 2010 Jul;139(1):270-80. doi: 10.1053/j.gastro.2010.04.003. Epub 2010 Apr 14. Gastroenterology. 2010. PMID: 20398665 Free PMC article.
-
Loss of complex O-glycosylation impairs exocrine pancreatic function and induces MODY8-like diabetes in mice.Exp Mol Med. 2018 Oct 10;50(10):1-13. doi: 10.1038/s12276-018-0157-3. Exp Mol Med. 2018. PMID: 30305605 Free PMC article.
-
First evidence of the interaction between deleted in malignant brain tumor 1 and galectin-3 in the mammalian oviduct.Histochem Cell Biol. 2014 Feb;141(2):181-90. doi: 10.1007/s00418-013-1145-2. Epub 2013 Sep 25. Histochem Cell Biol. 2014. PMID: 24065275
-
Extensive molecular analysis suggested the strong genetic heterogeneity of idiopathic chronic pancreatitis.Mol Med. 2016 Sep;22:300-309. doi: 10.2119/molmed.2016.00010. Epub 2016 May 26. Mol Med. 2016. PMID: 27264265 Free PMC article.
-
Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis.Theranostics. 2018 Feb 7;8(6):1607-1623. doi: 10.7150/thno.22958. eCollection 2018. Theranostics. 2018. PMID: 29556344 Free PMC article.
References
-
- Arvan P, Zhang BY, Feng LJ, Liu M, Kuliawat R. Lumenal protein multimerization in the distal secretory pathway/secretory granules. Curr Opin Cell Biol. 2002;14:448–453. - PubMed
-
- Blackburn AC, Hill LZ, Roberts AL, Wang J, Aud D, Jung J, Nikolcheva T, Allard J, Peltz G, Otis CN, Cao QJ, Ricketts RS, Naber SP, Mollenhauer J, Poustka A, Malamud D, Jerry DJ. Genetic mapping in mice identifies DMBT1 as a candidate modifier of mammary tumors and breast cancer risk. Am J Pathol. 2007;170:2030–2041. - PMC - PubMed
-
- Bodenteich A, Chissoe S, Wang YF, Roe BA. Shotgun Cloning as the Strategy of Choice to Generate Templates for High-throughput Dideoxynucleotide Sequencing. In: Venter JC, editor. Automated DNA Sequencing and Analysis Techniques. London: Academic Press; 1993. pp. 42–50.
-
- Boulatnikov I, De Lisle RC. Binding of the Golgi sorting receptor Muclin to pancreatic zymogens through sulfated O-linked oligosaccharides. J Biol Chem. 2004;279:40918–40926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

