Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation
- PMID: 18202122
- PMCID: PMC2329272
- DOI: 10.1210/en.2007-0582
Biochemical and structural characterization of apolipoprotein A-I binding protein, a novel phosphoprotein with a potential role in sperm capacitation
Abstract
The physiological changes that sperm undergo in the female reproductive tract rendering them fertilization-competent constitute the phenomenon of capacitation. Cholesterol efflux from the sperm surface and protein kinase A (PKA)-dependent phosphorylation play major regulatory roles in capacitation, but the link between these two phenomena is unknown. We report that apolipoprotein A-I binding protein (AI-BP) is phosphorylated downstream to PKA activation, localizes to both sperm head and tail domains, and is released from the sperm into the media during in vitro capacitation. AI-BP interacts with apolipoprotein A-I, the component of high-density lipoprotein involved in cholesterol transport. The crystal structure demonstrates that the subunit of the AI-BP homodimer has a Rossmann-like fold. The protein surface has a large two compartment cavity lined with conserved residues. This cavity is likely to constitute an active site, suggesting that AI-BP functions as an enzyme. The presence of AI-BP in sperm, its phosphorylation by PKA, and its release during capacitation suggest that AI-BP plays an important role in capacitation possibly providing a link between protein phosphorylation and cholesterol efflux.
Figures


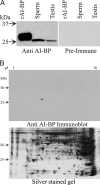






References
-
- Austin CR 1952 The capacitation of the mammalian sperm. Nature 170:326 - PubMed
-
- Yanagimachi R 1994 Mammalian fertilization. 2nd ed. New York: Raven Press
-
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB 2002 Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 53:133–150 - PubMed
-
- Jha KN, Kameshwari DB, Shivaji S 2003 Role of signaling pathways in regulating the capacitation of mammalian spermatozoa. Cell Mol Biol 49:329–340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

