Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction
- PMID: 18202178
- PMCID: PMC2234135
- DOI: 10.1073/pnas.0709739105
Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction
Abstract
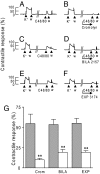
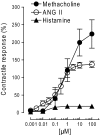
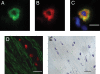
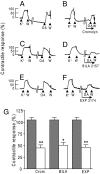
We previously reported that mast cells express renin, the rate-limiting enzyme in the renin-angiotensin cascade. We have now assessed whether mast cell renin release triggers angiotensin formation in the airway. In isolated rat bronchial rings, mast cell degranulation released enzyme with angiotensin I-forming activity blocked by the selective renin inhibitor BILA2157. Local generation of angiotensin (ANG II) from mast cell renin elicited bronchial smooth muscle contraction mediated by ANG II type 1 receptors (AT(1)R). In a guinea pig model of immediate type hypersensitivity, anaphylactic mast cell degranulation in bronchial rings resulted in ANG II-mediated constriction. As in rat bronchial rings, bronchoconstriction (BC) was inhibited by a renin inhibitor, an AT(1)R blocker, and a mast cell stabilizer. Anaphylactic release of renin, histamine, and beta-hexosaminidase from mast cells was confirmed in the effluent from isolated, perfused guinea pig lung. To relate the significance of this finding to humans, mast cells were isolated from macroscopically normal human lung waste tissue specimens. Sequence analysis of human lung mast cell RNA showed 100% homology between human lung mast cell renin and kidney renin between exons 1 and 10. Furthermore, the renin protein expressed in lung mast cells was enzymatically active. Our results demonstrate the existence of an airway renin-angiotensin system triggered by release of mast-cell renin. The data show that locally produced ANG II is a critical factor governing BC, opening the possibility for novel therapeutic targets in the management of airway disease.
Conflict of interest statement
Conflict of interest statement: A patent for related technology has been filed with the U.S. Patent and Trademark office by Cornell University. R.B.S. and R.L. are coinventors.
Figures

Similar articles
-
Mast cell degranulation mediates bronchoconstriction via serotonin and not via renin release.Eur J Pharmacol. 2010 Aug 25;640(1-3):185-9. doi: 10.1016/j.ejphar.2010.04.058. Epub 2010 May 10. Eur J Pharmacol. 2010. PMID: 20462506
-
Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion.J Clin Invest. 2006 Apr;116(4):1063-70. doi: 10.1172/JCI25713. J Clin Invest. 2006. PMID: 16585966 Free PMC article.
-
Mast cells are required for the development of renal fibrosis in the rodent unilateral ureteral obstruction model.Am J Physiol Renal Physiol. 2012 Jan 1;302(1):F192-204. doi: 10.1152/ajprenal.00562.2010. Epub 2011 Sep 28. Am J Physiol Renal Physiol. 2012. PMID: 21957176 Free PMC article.
-
Targeting cardiac mast cells: pharmacological modulation of the local renin-angiotensin system.Curr Pharm Des. 2011 Nov;17(34):3744-52. doi: 10.2174/138161211798357908. Curr Pharm Des. 2011. PMID: 22103845 Free PMC article. Review.
-
Recent Updates on the Proximal Tubule Renin-Angiotensin System in Angiotensin II-Dependent Hypertension.Curr Hypertens Rep. 2016 Aug;18(8):63. doi: 10.1007/s11906-016-0668-z. Curr Hypertens Rep. 2016. PMID: 27372447 Free PMC article. Review.
Cited by
-
Enalapril mitigates focal alveolar lesions, a histological marker of late pulmonary injury by radiation to the lung.Radiat Res. 2013 Apr;179(4):465-74. doi: 10.1667/RR3127.1. Epub 2013 Mar 12. Radiat Res. 2013. PMID: 23480564 Free PMC article.
-
Angiotensin II induces hyperresponsiveness of bronchial smooth muscle via an activation of p42/44 ERK in rats.Pflugers Arch. 2010 Aug;460(3):645-55. doi: 10.1007/s00424-010-0844-y. Epub 2010 May 22. Pflugers Arch. 2010. PMID: 20495822
-
Plant-Derived Antioxidants for Management of COVID-19: A Comprehensive Review of Molecular Mechanisms.Tanaffos. 2023 Jan;22(1):27-39. Tanaffos. 2023. PMID: 37920320 Free PMC article. Review.
-
Renin released from mast cells activated by circulating MCP-1 initiates the microvascular phase of the systemic inflammation of alveolar hypoxia.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2264-70. doi: 10.1152/ajpheart.00461.2011. Epub 2011 Sep 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21963836 Free PMC article.
-
E-NTPDase1/CD39 modulates renin release from heart mast cells during ischemia/reperfusion: a novel cardioprotective role.FASEB J. 2015 Jan;29(1):61-9. doi: 10.1096/fj.14-261867. Epub 2014 Oct 15. FASEB J. 2015. PMID: 25318477 Free PMC article.
References
-
- Valtin H, Schafer JA. Renal Function. Boston: Little, Brown; 1995. pp. 135–138.
-
- Phillips MI, Speakman EA, Kimura B. Regul Pept. 1993;43:1–20. - PubMed
-
- Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T, McAnulty RJ, Laurent GJ. Am J Physiol. 2004;286:L156–L164. - PubMed
-
- Campbell DJ, Kladis A, Duncan AM. Hypertension. 1993;22:513–522. - PubMed
-
- Campbell DJ, Kladis A, Valentijn AJ. J Cardiovasc Pharmacol. 1995;26:233–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

