The COUP-TFs compose a family of functionally related transcription factors
- PMID: 1820218
- PMCID: PMC5952191
The COUP-TFs compose a family of functionally related transcription factors
Abstract
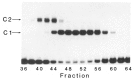
The chicken ovalbumin upstream promoter transcription factors (COUP-TFs) are members of the steroid/thyroid hormone receptor superfamily and function in transcriptional regulation of a wide variety of genes. The COUP-TFs purified from HeLa nuclear extract by COUP-affinity chromatography are composed of multiple M(r) forms. The Low M(r) COUP-TFs (43,000, 44,000, 46,000, and 47,000 M(r)) produce a relatively fast migrating complex (C1) with DNA in electrophoresis mobility shift assays, while the high M(r) forms (66,000, 68,000, 72,000, and 74,000 M(r)) produce a slower migrating (C2) complex. The high M(r) COUP-TFs were purified by gel filtration chromatography and independently formed the C2 DNA complex, probably acting as dimers. The high M(r) forms are indistinguishable from the low M(r) COUP-TFs in DNA binding and in enhancement of in vitro transcription from the ovalbumin promoter. The finding of multiple COUP-TF forms led us to clone a second low M(r) COUP-TF, "COUP-TF2." The COUP-TF2 sequence has very strong homology with COUP-TF1. The N-termini of COUP-TF1 and COUP-TF2 are least similar, but both contain glutamine-rich and proline-rich motifs, putative activation domains.
Figures





References
-
- Beato M. (1989), Cell 56, 335–344. - PubMed
-
- Berger S. L., Cress W. D., Cress A., Triezenberg S. J., and Guarente L. (1990), Cell 61, 1199–1208. - PubMed
-
- Cooney A. J., Tsai S. Y., O’Malley B. W., and Tsai M.-J. (1991), J Virology (in press).
-
- Courey A. J., Holtzman D. A., Jackson S. P., and Tjian R. (1989), Cell 59, 827–836. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
