Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish
- PMID: 18202183
- PMCID: PMC2234125
- DOI: 10.1073/pnas.0704963105
Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish
Abstract
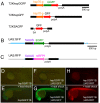
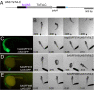
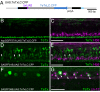
Targeted gene expression is a powerful approach to study the function of genes and cells in vivo. In Drosophila, the P element-mediated Gal4-UAS method has been successfully used for this purpose. However, similar methods have not been established in vertebrates. Here we report the development of a targeted gene expression methodology in zebrafish based on the Tol2 transposable element and its application to the functional study of neural circuits. First, we developed gene trap and enhancer trap constructs carrying an engineered yeast Gal4 transcription activator (Gal4FF) and transgenic reporter fish carrying the GFP or the RFP gene downstream of the Gal4 recognition sequence (UAS) and showed that the Gal4FF can activate transcription through UAS in zebrafish. Second, by using this Gal4FF-UAS system, we performed large-scale screens and generated a large collection of fish lines that expressed Gal4FF in specific tissues, cells, and organs. Finally, we developed transgenic effector fish carrying the tetanus toxin light chain (TeTxLC) gene downstream of UAS, which is known to block synaptic transmission. We crossed the Gal4FF fish with the UAS:TeTxLC fish and analyzed double transgenic embryos for defects in touch response. From this analysis, we discovered that targeted expression of TeTxLC in distinct populations of neurons in the brain and the spinal cord caused distinct abnormalities in the touch response behavior. These studies illustrate that our Gal4FF gene trap and enhancer trap methods should be an important resource for genetic analysis of neuronal functions and behavior in vertebrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Schiavo G, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. - PubMed
-
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. - PubMed
-
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–158. - PubMed
-
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

