Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells
- PMID: 18202186
- PMCID: PMC2312365
- DOI: 10.2353/ajpath.2008.070696
Regulation of Cripto-1 signaling and biological activity by caveolin-1 in mammary epithelial cells
Abstract
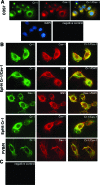
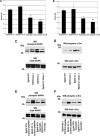
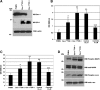
Human and mouse Cripto-1 (CR-1/Cr-1) proteins play an important role in mammary gland development and tumorigenesis. In this study, we examined the relationship between Cripto-1 and caveolin-1 (Cav-1), a membrane protein that acts as a tumor suppressor in the mammary gland. Cripto-1 was found to interact with Cav-1 in COS7 cells and mammary epithelial cells. Using EpH4 mouse mammary epithelial cells expressing Cr-1 (EpH4 Cr-1) or Cr-1 and Cav-1 (EpH4 Cr-1/Cav-1), we demonstrate that Cav-1 expression markedly reduced the ability of Cr-1 to enhance migration, invasion, and formation of branching structures in EpH4 Cr-1/Cav-1 cells as compared to EpH4 Cr-1 cells. Furthermore, coexpression of Cav-1 together with Cr-1 in EpH4 Cr-1/Cav-1 cells inhibited Cr-1-mediated activation of c-src and mitogen-activated protein kinase signaling pathways. Conversely, primary mammary epithelial cells isolated from Cav-1 null(-/-)/mouse mammary tumor virus-CR-1 transgenic animals showed enhanced motility and activation of mitogen-activated protein kinase and c-src as compared to Cav-1(+/-)/CR-1 mammary cells. Finally, mammary tumors derived from mouse mammary tumor virus-CR-1 mice showed a dramatic reduction of Cav-1 expression as compared to mammary tissue from normal FVB/N mice, suggesting that in vivo Cav-1 is down-regulated during the process of CR-1-mediated mammary tumorigenesis.
Figures





Similar articles
-
Netrin-1 regulates invasion and migration of mouse mammary epithelial cells overexpressing Cripto-1 in vitro and in vivo.J Cell Sci. 2005 Oct 15;118(Pt 20):4633-43. doi: 10.1242/jcs.02574. Epub 2005 Sep 21. J Cell Sci. 2005. PMID: 16176936
-
Msx2 induces epithelial-mesenchymal transition in mouse mammary epithelial cells through upregulation of Cripto-1.J Cell Physiol. 2009 Jun;219(3):659-66. doi: 10.1002/jcp.21712. J Cell Physiol. 2009. PMID: 19170109 Free PMC article.
-
Detection and localization of Cripto-1 binding in mouse mammary epithelial cells and in the mouse mammary gland using an immunoglobulin-cripto-1 fusion protein.J Cell Physiol. 2002 Jan;190(1):74-82. doi: 10.1002/jcp.10037. J Cell Physiol. 2002. PMID: 11807813
-
The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer.Endocr Relat Cancer. 2000 Dec;7(4):199-226. doi: 10.1677/erc.0.0070199. Endocr Relat Cancer. 2000. PMID: 11174844 Review.
-
Cripto: a novel epidermal growth factor (EGF)-related peptide in mammary gland development and neoplasia.Bioessays. 1999 Jan;21(1):61-70. doi: 10.1002/(SICI)1521-1878(199901)21:1<61::AID-BIES8>3.0.CO;2-H. Bioessays. 1999. PMID: 10070255 Review.
Cited by
-
Cripto/GRP78 modulation of the TGF-β pathway in development and oncogenesis.FEBS Lett. 2012 Jul 4;586(14):1836-45. doi: 10.1016/j.febslet.2012.01.051. Epub 2012 Feb 1. FEBS Lett. 2012. PMID: 22306319 Free PMC article. Review.
-
Advanced Lab-on-Fiber Optrodes Assisted by Oriented Antibody Immobilization Strategy.Biosensors (Basel). 2022 Nov 17;12(11):1040. doi: 10.3390/bios12111040. Biosensors (Basel). 2022. PMID: 36421158 Free PMC article.
-
Cripto-1 modulates macrophage cytokine secretion and phagocytic activity via NF-κB signaling.Immunol Res. 2016 Feb;64(1):104-14. doi: 10.1007/s12026-015-8724-3. Immunol Res. 2016. PMID: 26476731
-
Regulation of human Cripto-1 expression by nuclear receptors and DNA promoter methylation in human embryonal and breast cancer cells.J Cell Physiol. 2013 Jun;228(6):1174-88. doi: 10.1002/jcp.24271. J Cell Physiol. 2013. PMID: 23129342 Free PMC article.
-
The multifaceted role of the embryonic gene Cripto-1 in cancer, stem cells and epithelial-mesenchymal transition.Semin Cancer Biol. 2014 Dec;29:51-8. doi: 10.1016/j.semcancer.2014.08.003. Epub 2014 Aug 19. Semin Cancer Biol. 2014. PMID: 25153355 Free PMC article. Review.
References
-
- Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. - PubMed
-
- Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24:5731–5741. - PubMed
-
- Bianco C, Normanno N, Salomon DS, Ciardiello F. Role of the cripto (EGF-CFC) family in embryogenesis and cancer. Growth Factors. 2004;22:133–139. - PubMed
-
- Salomon DS, Bianco C, Ebert AD, Khan NI, De Santis M, Normanno N, Wechselberger C, Seno M, Williams K, Sanicola M, Foley S, Gullick WJ, Persico G. The EGF-CFC family: novel epidermal growth factor-related proteins in development and cancer. Endocr Relat Cancer. 2000;7:199–226. - PubMed
-
- Minchiotti G, Parisi S, Liguori G, Signore M, Lania G, Adamson ED, Lago CT, Persico MG. Membrane-anchorage of Cripto protein by glycosylphosphatidylinositol and its distribution during early mouse development. Mech Dev. 2000;90:133–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

