Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function
- PMID: 18202191
- PMCID: PMC2312371
- DOI: 10.2353/ajpath.2008.070870
Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function
Abstract
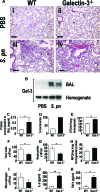
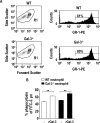
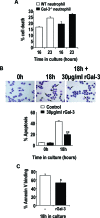
The Gram-positive Streptococcus pneumoniae is the leading cause of community-acquired pneumonia worldwide, resulting in high mortality. Our in vivo studies show that galectin-3(-/-) mice develop more severe pneumonia after infection with S. pneumoniae, as demonstrated by increased bacteremia and lung damage compared to wild-type mice and that galectin-3 reduces the severity of pneumococcal pneumonia in part by augmenting neutrophil function. Specifically, we show that 1) galectin-3 directly acts as a neutrophil-activating agent and potentiates the effect of fMLP, 2) exogenous galectin-3 augments neutrophil phagocytosis of bacteria and delays neutrophil apoptosis, 3) phagocytosis of apoptotic neutrophils by galectin-3(-/-) macrophages is less efficient compared to wild type, and 4) galectin-3 demonstrates bacteriostatic properties against S. pneumoniae in vitro. Furthermore, ad-back of recombinant galectin-3 in vivo protects galectin-3-deficient mice from developing severe pneumonia. Together, these results demonstrate that galectin-3 is a key molecule in the host defense against pneumococcal infection. Therapeutic strategies designed to augment galectin-3 activity may both enhance inflammatory cell function (by directly affecting neutrophil responsiveness and prolonging neutrophil longevity) and have direct bacteriostatic activity, improving clinical outcomes after severe pneumococcal infection.
Figures




References
-
- Paterson GK, Blue CE, Mitchell TJ. Role of interleukin-18 in experimental infections with Streptococcus pneumoniae. J Med Microbiol. 2005;54:323–326. - PubMed
-
- Feldman C. Clinical relevance of antimicrobial resistance in the management of pneumococcal community-acquired pneumonia. J Lab Clin Med. 2004;143:269–283. - PubMed
-
- Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. - PubMed
-
- Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. - PubMed
-
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

