Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease
- PMID: 18202196
- PMCID: PMC2312356
- DOI: 10.2353/ajpath.2008.070293
Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease
Abstract
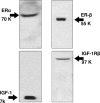
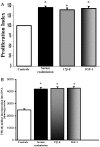
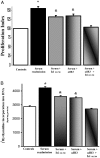
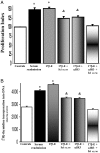
We evaluated the morphological and functional features of hepatic cyst epithelium in adult autosomal dominant polycystic kidney disease (ADPKD). In six ADPKD patients, we investigated the morphology of cyst epithelium apical surface by scanning electron microscopy and the expression of estrogen receptors (ERs), insulin-like growth factor 1 (IGF1), IGF1 receptors (IGF1-R), growth hormone receptor, the proliferation marker proliferating cell nuclear antigen, and pAKT by immunohistochemistry and immunofluorescence. Proliferation of liver cyst-derived epithelial cells was evaluated by both MTS proliferation assay and [(3)H]thymidine incorporation into DNA. The hepatic cyst epithelium displayed heterogeneous features, being normal in small cysts (<1 cm), characterized by rare or shortened cilia in 1- to 3-cm cysts, and exhibiting the absence of both primary cilia and microvilli in large cysts (>3 cm). Cyst epithelium showed marked immunohistochemical expression of ER, growth hormone receptor, IGF1, IGF1-R, proliferating cell nuclear antigen, and pAKT. IGF1 was 10-fold more enriched in the hepatic cyst fluid than in serum. Serum-deprived liver cyst-derived epithelial cells proliferated when exposed to 17beta-estradiol and IGF1 and when exposed to human cyst fluid. ER or IGF1-R antagonists inhibited the proliferative effect of serum readmission, cyst fluid, 17beta-estradiol, and IGF1. Our findings could explain the role of estrogens in accelerating the progression of ADPKD and may suggest a potential benefit of therapeutic strategies based on estrogen antagonism.
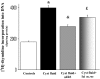
Figures






Similar articles
-
Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice.Hepatology. 2010 May;51(5):1778-88. doi: 10.1002/hep.23511. Hepatology. 2010. PMID: 20131403 Free PMC article.
-
Secretion of cytokines and growth factors into autosomal dominant polycystic kidney disease liver cyst fluid.Hepatology. 2004 Oct;40(4):836-46. doi: 10.1002/hep.20401. Hepatology. 2004. PMID: 15382115
-
Identifying cystogenic paracrine signaling molecules in cyst fluid of patients with polycystic kidney disease.Am J Physiol Renal Physiol. 2019 Jan 1;316(1):F204-F213. doi: 10.1152/ajprenal.00470.2018. Epub 2018 Nov 7. Am J Physiol Renal Physiol. 2019. PMID: 30403162
-
Polycystic liver diseases.Dig Liver Dis. 2010 Apr;42(4):261-71. doi: 10.1016/j.dld.2010.01.006. Epub 2010 Feb 6. Dig Liver Dis. 2010. PMID: 20138815 Free PMC article. Review.
-
Hepatic cyst infection in autosomal dominant polycystic kidney disease.Mayo Clin Proc. 1990 Jul;65(7):933-42. doi: 10.1016/s0025-6196(12)65154-4. Mayo Clin Proc. 1990. PMID: 2198396 Review.
Cited by
-
Genetics, pathobiology and therapeutic opportunities of polycystic liver disease.Nat Rev Gastroenterol Hepatol. 2022 Sep;19(9):585-604. doi: 10.1038/s41575-022-00617-7. Epub 2022 May 13. Nat Rev Gastroenterol Hepatol. 2022. PMID: 35562534 Review.
-
Autosomal dominant polycystic kidney disease: the last 3 years.Kidney Int. 2009 Jul;76(2):149-68. doi: 10.1038/ki.2009.128. Epub 2009 May 20. Kidney Int. 2009. PMID: 19455193 Free PMC article. Review.
-
Isolated polycystic liver disease.Adv Chronic Kidney Dis. 2010 Mar;17(2):181-9. doi: 10.1053/j.ackd.2009.12.005. Adv Chronic Kidney Dis. 2010. PMID: 20219621 Free PMC article. Review.
-
A TGFβ-ECM-integrin signaling axis drives structural reconfiguration of the bile duct to promote polycystic liver disease.Sci Transl Med. 2023 Sep 13;15(713):eabq5930. doi: 10.1126/scitranslmed.abq5930. Epub 2023 Sep 13. Sci Transl Med. 2023. PMID: 37703354 Free PMC article.
-
Close pathological correlations between chronic kidney disease and reproductive organ-associated abnormalities in female cotton rats.Exp Biol Med (Maywood). 2018 Mar;243(5):418-427. doi: 10.1177/1535370218758250. Epub 2018 Feb 7. Exp Biol Med (Maywood). 2018. PMID: 29412002 Free PMC article.
References
-
- Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. - PubMed
-
- Ramos A, Torres VE, Holley KE, Offord KP, Rakela J, Ludwig J. The liver in autosomal dominant polycystic kidney disease. Implications for pathogenesis. Arch Pathol Lab Med. 1990;114:180–184. - PubMed
-
- Everson GT. Hepatic cysts in autosomal dominant polycystic kidney disease. Mayo Clin Proc. 1990;65:1020–1025. - PubMed
-
- Arnold HL, Harrison SA. New advances in evaluation and management of patients with polycystic liver disease. Am J Gastroenterol. 2005;100:2569–2582. - PubMed
-
- Everson GT, Emmett M, Brown WR, Redmond P, Thickman D. Functional similarities of hepatic cystic and biliary epithelium: studies of fluid constituents and in vivo secretion in response to secretin. Hepatology. 1990;11:557–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

