Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation
- PMID: 18202223
- PMCID: PMC2275010
- DOI: 10.1182/blood-2007-11-122119
Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation
Abstract
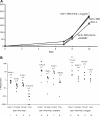
Hematopoietic stem cells (HSCs) are the basis of bone marrow transplantation and are attractive target cells for hematopoietic gene therapy, but these important clinical applications have been severely hampered by difficulties in ex vivo expansion of HSCs. In particular, the use of cord blood for adult transplantation is greatly limited by the number of HSCs. Previously we identified angiopoietin-like proteins and IGF-binding protein 2 (IGFBP2) as new hormones that, together with other factors, can expand mouse bone marrow HSCs in culture. Here, we measure the activity of multipotent human severe combined immunodeficient (SCID)-repopulating cells (SRCs) by transplantation into the nonobese diabetic SCID (NOD/SCID) mice; secondary transplantation was performed to evaluate the self-renewal potential of SRCs. A serum-free medium containing SCF, TPO, and FGF-1 or Flt3-L cannot significantly support expansion of the SRCs present in human cord blood CD133+ cells. Addition of either angiopoietin-like 5 or IGF-binding protein 2 to the cultures led to a sizable expansion of HSC numbers, as assayed by NOD/SCID transplantation. A serum-free culture containing SCF, TPO, FGF-1, angiopoietin-like 5, and IGFBP2 supports an approximately 20-fold net expansion of repopulating human cord blood HSCs, a number potentially applicable to several clinical processes including HSC transplantation.
Figures

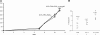


Similar articles
-
Low-dose insulin-like growth factor binding proteins 1 and 2 and angiopoietin-like protein 3 coordinately stimulate ex vivo expansion of human umbilical cord blood hematopoietic stem cells as assayed in NOD/SCID gamma null mice.Stem Cell Res Ther. 2014 May 30;5(3):71. doi: 10.1186/scrt460. Stem Cell Res Ther. 2014. PMID: 24886724 Free PMC article.
-
Comparison of Different Cytokine Conditions Reveals Resveratrol as a New Molecule for Ex Vivo Cultivation of Cord Blood-Derived Hematopoietic Stem Cells.Stem Cells Transl Med. 2015 Sep;4(9):1064-72. doi: 10.5966/sctm.2014-0284. Epub 2015 Jul 9. Stem Cells Transl Med. 2015. PMID: 26160960 Free PMC article.
-
Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential.Stem Cells Dev. 2011 Aug;20(8):1371-81. doi: 10.1089/scd.2010.0456. Epub 2011 Jan 31. Stem Cells Dev. 2011. PMID: 21142526 Free PMC article.
-
Ex vivo expansion of hematopoietic stem cells.Sci China Life Sci. 2015 Sep;58(9):839-53. doi: 10.1007/s11427-015-4895-3. Epub 2015 Aug 5. Sci China Life Sci. 2015. PMID: 26246379 Review.
-
Concise review: ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: basic principles, experimental approaches, and impact in regenerative medicine.Stem Cells Transl Med. 2013 Nov;2(11):830-8. doi: 10.5966/sctm.2013-0071. Epub 2013 Oct 7. Stem Cells Transl Med. 2013. PMID: 24101670 Free PMC article. Review.
Cited by
-
The Ongoing Challenge of Hematopoietic Stem Cell-Based Gene Therapy for β-Thalassemia.Stem Cells Int. 2011;2011:987980. doi: 10.4061/2011/987980. Epub 2011 Nov 13. Stem Cells Int. 2011. PMID: 22190966 Free PMC article.
-
Engineered hematopoietic and immune cells derived from human pluripotent stem cells.Exp Hematol. 2023 Nov;127:14-27. doi: 10.1016/j.exphem.2023.08.006. Epub 2023 Aug 22. Exp Hematol. 2023. PMID: 37611730 Free PMC article. Review.
-
New relationships of human hematopoietic lineages facilitate detection of multipotent hematopoietic stem and progenitor cells.Cell Cycle. 2013 Nov 15;12(22):3478-82. doi: 10.4161/cc.26900. Epub 2013 Oct 23. Cell Cycle. 2013. PMID: 24189527 Free PMC article.
-
Ex vivo expansion of human hematopoietic stem and progenitor cells.Blood. 2011 Jun 9;117(23):6083-90. doi: 10.1182/blood-2011-01-283606. Epub 2011 Mar 23. Blood. 2011. PMID: 21436068 Free PMC article. Review.
-
Low-dose insulin-like growth factor binding proteins 1 and 2 and angiopoietin-like protein 3 coordinately stimulate ex vivo expansion of human umbilical cord blood hematopoietic stem cells as assayed in NOD/SCID gamma null mice.Stem Cell Res Ther. 2014 May 30;5(3):71. doi: 10.1186/scrt460. Stem Cell Res Ther. 2014. PMID: 24886724 Free PMC article.
References
-
- Sauvageau G, Iscove NN, Humphries RK. In vitro and in vivo expansion of hematopoietic stem cells. Oncogene. 2004;23:7223–7232. - PubMed
-
- Kirouac DC, Zandstra PW. Understanding cellular networks to improve hematopoietic stem cell expansion cultures. Curr Opin Biotechnol. 2006;17:538–547. - PubMed
-
- Brunstein CG, Wagner JE. Umbilical cord blood transplantation and banking. Annu Rev Med. 2006;57:403–417. - PubMed
-
- Robinson S, Niu T, de Lima M, et al. Ex vivo expansion of umbilical cord blood. Cytotherapy. 2005;7:243–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

