Identification of cardiac-specific myosin light chain kinase
- PMID: 18202317
- PMCID: PMC2504503
- DOI: 10.1161/CIRCRESAHA.107.161687
Identification of cardiac-specific myosin light chain kinase
Abstract
Two myosin light chain (MLC) kinase (MLCK) proteins, smooth muscle (encoded by mylk1 gene) and skeletal (encoded by mylk2 gene) MLCK, have been shown to be expressed in mammals. Even though phosphorylation of its putative substrate, MLC2, is recognized as a key regulator of cardiac contraction, a MLCK that is preferentially expressed in cardiac muscle has not yet been identified. In this study, we characterized a new kinase encoded by a gene homologous to mylk1 and -2, named cardiac MLCK, which is specifically expressed in the heart in both atrium and ventricle. In fact, expression of cardiac MLCK is highly regulated by the cardiac homeobox protein Nkx2-5 in neonatal cardiomyocytes. The overall structure of cardiac MLCK protein is conserved with skeletal and smooth muscle MLCK; however, the amino terminus is quite unique, without significant homology to other known proteins, and its catalytic activity does not appear to be regulated by Ca(2+)/calmodulin in vitro. Cardiac MLCK is phosphorylated and the level of phosphorylation is increased by phenylephrine stimulation accompanied by increased level of MLC2v phosphorylation. Both overexpression and knockdown of cardiac MLCK in cultured cardiomyocytes revealed that cardiac MLCK is likely a new regulator of MLC2 phosphorylation, sarcomere organization, and cardiomyocyte contraction.
Figures




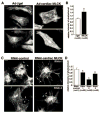
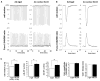

Comment in
-
Cardiac myosin light chain kinase: a new player in the regulation of myosin light chain in the heart.Circ Res. 2008 Mar 14;102(5):516-8. doi: 10.1161/CIRCRESAHA.108.173005. Circ Res. 2008. PMID: 18340016 No abstract available.
Similar articles
-
A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart.J Clin Invest. 2007 Oct;117(10):2812-24. doi: 10.1172/JCI30804. J Clin Invest. 2007. PMID: 17885681 Free PMC article.
-
β-adrenergic activation may promote myosin light chain kinase degradation through calpain in pressure overload-induced cardiac hypertrophy: β-adrenergic activation results in MLCK degradation.Biomed Pharmacother. 2020 Sep;129:110438. doi: 10.1016/j.biopha.2020.110438. Epub 2020 Jul 4. Biomed Pharmacother. 2020. PMID: 32768940
-
Key role of myosin light chain (MLC) kinase-mediated MLC2a phosphorylation in the alpha 1-adrenergic positive inotropic effect in human atrium.Cardiovasc Res. 2005 Jan 1;65(1):211-20. doi: 10.1016/j.cardiores.2004.09.019. Cardiovasc Res. 2005. PMID: 15621049
-
Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle.Arch Biochem Biophys. 2011 Jun 15;510(2):120-8. doi: 10.1016/j.abb.2011.01.017. Epub 2011 Feb 1. Arch Biochem Biophys. 2011. PMID: 21284933 Free PMC article. Review.
-
Phosphorylation of myosin regulatory light chain by myosin light chain kinase, and muscle contraction.Circ J. 2009 Feb;73(2):208-13. doi: 10.1253/circj.cj-08-1041. Epub 2008 Dec 26. Circ J. 2009. PMID: 19110504 Review.
Cited by
-
Role of myosin light chain phosphatase in cardiac physiology and pathophysiology.J Mol Cell Cardiol. 2016 Dec;101:35-43. doi: 10.1016/j.yjmcc.2016.10.004. Epub 2016 Oct 11. J Mol Cell Cardiol. 2016. PMID: 27742556 Free PMC article. Review.
-
Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease.Gene. 2015 Sep 10;569(1):14-20. doi: 10.1016/j.gene.2015.06.027. Epub 2015 Jun 12. Gene. 2015. PMID: 26074085 Free PMC article. Review.
-
A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain.Mol Cell Proteomics. 2010 Sep;9(9):1804-18. doi: 10.1074/mcp.M110.000075. Epub 2010 May 5. Mol Cell Proteomics. 2010. PMID: 20445002 Free PMC article.
-
Regulation of myosin light-chain phosphorylation and its roles in cardiovascular physiology and pathophysiology.Hypertens Res. 2022 Jan;45(1):40-52. doi: 10.1038/s41440-021-00733-y. Epub 2021 Oct 6. Hypertens Res. 2022. PMID: 34616031 Review.
-
Targeted myocardial gene expression in failing hearts by RNA sequencing.J Transl Med. 2016 Nov 25;14(1):327. doi: 10.1186/s12967-016-1083-6. J Transl Med. 2016. PMID: 27884156 Free PMC article.
References
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. New York: Garland Publishing Inc; 2002.
-
- Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. - PubMed
-
- Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–4530. - PubMed
-
- Moss RL, Fitzsimons DP. Myosin light chain 2 into the mainstream of cardiac development and contractility. Circ Res. 2006;99:225–227. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

