Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan
- PMID: 18202348
- PMCID: PMC2720118
- DOI: 10.1164/rccm.200708-1271OC
Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan
Abstract
Rationale: Acquired pulmonary alveolar proteinosis (PAP) is a syndrome characterized by pulmonary surfactant accumulation occurring in association with granulocyte/macrophage colony-stimulating factor autoantibodies (autoimmune PAP) or as a consequence of another disease (secondary PAP). Because PAP is rare, prior reports were based on limited patient numbers or a synthesis of historical data.
Objectives: To describe the epidemiologic, clinical, physiologic, and laboratory features of autoimmune PAP in a large, contemporaneous cohort of patients with PAP.
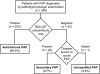
Methods: Over 6 years, 248 patients with PAP were enrolled in a Japanese national registry, including 223 with autoimmune PAP.
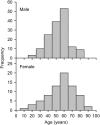
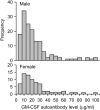
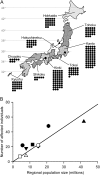
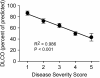
Measurements and main results: Autoimmune PAP represented 89.9% of cases and had a minimum incidence and prevalence of 0.49 and 6.2 per million, respectively. The male to female ratio was 2.1:1, and the median age at diagnosis was 51 years. A history of smoking occurred in 56%, and dust exposure occurred in 23%; instances of familial onset did not occur. Dyspnea was the most common presenting symptom, occurring in 54.3%. Importantly, 31.8% of patients were asymptomatic and were identified by health screening. Intercurrent illnesses, including infections, were infrequent. A disease severity score reflecting the presence of symptoms and degree of hypoxemia correlated well with carbon monoxide diffusing capacity and serum biomarkers, less well with pulmonary function, and not with granulocyte/macrophage colony-stimulating factor autoantibody levels or duration of disease.
Conclusions: Autoimmune PAP had an incidence and prevalence higher than previously reported and was not strongly linked to smoking, occupational exposure, or other illnesses. The disease severity score and biomarkers provide novel and potentially useful outcome measures in PAP.
Figures

References
-
- Rosen SG, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958;258:1123–1142. - PubMed
-
- Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 2002;166:215–235. - PubMed
-
- Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–2539. - PubMed
-
- Kavuru MS, Bonfield TL, Thompson MJ. Pulmonary alveolar proteinosis. In: Mason RJ, Broaddus VC, Murray JF, Nadel JA, editors. Textbook of pulmonary medicine, 4th ed. Philadelphia: Elsevier; 2006. pp. 1716–1734.
-
- Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet 2004;13:R207–R215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

