Regulation of serotonin biosynthesis by the G proteins Galphao and Galphaq controls serotonin signaling in Caenorhabditis elegans
- PMID: 18202365
- PMCID: PMC2206068
- DOI: 10.1534/genetics.107.079780
Regulation of serotonin biosynthesis by the G proteins Galphao and Galphaq controls serotonin signaling in Caenorhabditis elegans
Abstract
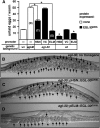
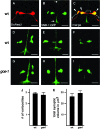
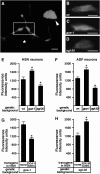
To analyze mechanisms that modulate serotonin signaling, we investigated how Caenorhabditis elegans regulates the function of serotonergic motor neurons that stimulate egg-laying behavior. Egg laying is inhibited by the G protein Galphao and activated by the G protein Galphaq. We found that Galphao and Galphaq act directly in the serotonergic HSN motor neurons to control egg laying. There, the G proteins had opposing effects on transcription of the tryptophan hydroxylase gene tph-1, which encodes the rate-limiting enzyme for serotonin biosynthesis. Antiserotonin staining confirmed that Galphao and Galphaq antagonistically affect serotonin levels. Altering tph-1 gene dosage showed that small changes in tph-1 expression were sufficient to affect egg-laying behavior. Epistasis experiments showed that signaling through the G proteins has additional tph-1-independent effects. Our results indicate that (1) serotonin signaling is regulated by modulating serotonin biosynthesis and (2) Galphao and Galphaq act in the same neurons to have opposing effects on behavior, in part, by antagonistically regulating transcription of specific genes. Galphao and Galphaq have opposing effects on many behaviors in addition to egg laying and may generally act, as they do in the egg-laying system, to integrate multiple signals and consequently set levels of transcription of genes that affect neurotransmitter release.
Figures




Similar articles
-
Presynaptic Gαo (GOA-1) signals to depress command neuron excitability and allow stretch-dependent modulation of egg laying in Caenorhabditis elegans.Genetics. 2021 Aug 9;218(4):iyab080. doi: 10.1093/genetics/iyab080. Genetics. 2021. PMID: 34037773 Free PMC article.
-
STR-33, a novel G protein-coupled receptor that regulates locomotion and egg laying in Caenorhabditis elegans.J Biol Chem. 2011 Nov 18;286(46):39860-70. doi: 10.1074/jbc.M111.241000. Epub 2011 Sep 21. J Biol Chem. 2011. PMID: 21937442 Free PMC article.
-
Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle.Genetics. 2003 Dec;165(4):1805-22. doi: 10.1093/genetics/165.4.1805. Genetics. 2003. PMID: 14704167 Free PMC article.
-
Neurotransmitter signaling through heterotrimeric G proteins: insights from studies in C. elegans.WormBook. 2018 Dec 11;2018:1-52. doi: 10.1895/wormbook.1.75.2. WormBook. 2018. PMID: 26937633 Free PMC article. Review.
-
Heterotrimeric G proteins in C. elegans.WormBook. 2006 Oct 13:1-25. doi: 10.1895/wormbook.1.75.1. WormBook. 2006. PMID: 18050432 Free PMC article. Review.
Cited by
-
IRK-1 potassium channels mediate peptidergic inhibition of Caenorhabditis elegans serotonin neurons via a G(o) signaling pathway.J Neurosci. 2012 Nov 14;32(46):16285-95. doi: 10.1523/JNEUROSCI.2667-12.2012. J Neurosci. 2012. PMID: 23152612 Free PMC article.
-
Nervous system-wide analysis of Hox regulation of terminal neuronal fate specification in Caenorhabditis elegans.PLoS Genet. 2022 Feb 28;18(2):e1010092. doi: 10.1371/journal.pgen.1010092. eCollection 2022 Feb. PLoS Genet. 2022. PMID: 35226663 Free PMC article.
-
Serotonin signals through postsynaptic Gαq, Trio RhoGEF, and diacylglycerol to promote Caenorhabditis elegans egg-laying circuit activity and behavior.Genetics. 2022 Jul 4;221(3):iyac084. doi: 10.1093/genetics/iyac084. Genetics. 2022. PMID: 35579369 Free PMC article.
-
C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog.Nat Neurosci. 2010 Jun;13(6):715-22. doi: 10.1038/nn.2540. Epub 2010 May 2. Nat Neurosci. 2010. PMID: 20436480 Free PMC article.
-
The serotonin circuit that coordinates germline proliferation and egg laying with other reproductive functions in Caenorhabditis elegans.Proc Biol Sci. 2022 Nov 30;289(1987):20220913. doi: 10.1098/rspb.2022.0913. Epub 2022 Nov 30. Proc Biol Sci. 2022. PMID: 36448283 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

