MEG-1 and MEG-2 are embryo-specific P-granule components required for germline development in Caenorhabditis elegans
- PMID: 18202375
- PMCID: PMC2206079
- DOI: 10.1534/genetics.107.080218
MEG-1 and MEG-2 are embryo-specific P-granule components required for germline development in Caenorhabditis elegans
Abstract
In Caenorhabditis elegans, germ granules called P granules are directly inherited from mother to daughter and segregate with the germ lineage as it separates from the soma during initial embryonic cell divisions. Here we define meg-1 and meg-2 (maternal-effect germ-cell defective), which are expressed in the maternal germline and encode proteins that localize exclusively to P granules during embryonic germline segregation. Localization of MEG-1 to P granules depends upon the membrane-bound protein MES-1. meg-1 mutants exhibit multiple germline defects: P-granule mis-segregation in embryos, underproliferation and aberrant P-granule morphology in larval germ cells, and ultimately, sterility as adults. The penetrance of meg-1 phenotypes increases when meg-2 is also absent. Loss of the P-granule component pgl-1 in meg-1 mutants increases germ-cell proliferation, while loss of glh-1 decreases proliferation. Because meg-1 is provided maternally but its action is required in the embryonic germ lineage during segregation from somatic lineages, it provides a critical link for ensuring the continuity of germline development from one generation to the next.
Figures
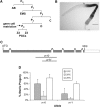
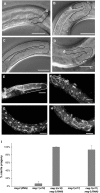
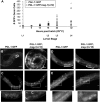
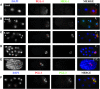
Similar articles
-
Selective autophagic degradation of maternally-loaded germline P granule components in somatic cells during C. elegans embryogenesis.Autophagy. 2009 Jul;5(5):717-9. doi: 10.4161/auto.5.5.8552. Epub 2009 Jul 26. Autophagy. 2009. PMID: 19372764
-
Genetic analysis of the Caenorhabditis elegans GLH family of P-granule proteins.Genetics. 2008 Apr;178(4):1973-87. doi: 10.1534/genetics.107.083469. Genetics. 2008. PMID: 18430929 Free PMC article.
-
Single-molecule dynamics of the P granule scaffold MEG-3 in the Caenorhabditis elegans zygote.Mol Biol Cell. 2019 Feb 1;30(3):333-345. doi: 10.1091/mbc.E18-06-0402. Epub 2018 Dec 12. Mol Biol Cell. 2019. PMID: 30540524 Free PMC article.
-
Connecting the Dots: Linking Caenorhabditis elegans Small RNA Pathways and Germ Granules.Trends Cell Biol. 2021 May;31(5):387-401. doi: 10.1016/j.tcb.2020.12.012. Epub 2021 Jan 29. Trends Cell Biol. 2021. PMID: 33526340 Review.
-
The diverse functions of germline P-granules in Caenorhabditis elegans.Mol Reprod Dev. 2013 Aug;80(8):624-31. doi: 10.1002/mrd.22136. Epub 2012 Dec 13. Mol Reprod Dev. 2013. PMID: 23150384 Review.
Cited by
-
Contrasting patterns of molecular evolution in metazoan germ line genes.BMC Evol Biol. 2019 Feb 11;19(1):53. doi: 10.1186/s12862-019-1363-x. BMC Evol Biol. 2019. PMID: 30744572 Free PMC article.
-
Sex-specific developmental gene expression atlas unveils dimorphic gene networks in C. elegans.Nat Commun. 2024 May 20;15(1):4273. doi: 10.1038/s41467-024-48369-z. Nat Commun. 2024. PMID: 38769103 Free PMC article.
-
Specialized germline P-bodies are required to specify germ cell fate in Caenorhabditis elegans embryos.Development. 2022 Nov 1;149(21):dev200920. doi: 10.1242/dev.200920. Epub 2022 Nov 7. Development. 2022. PMID: 36196602 Free PMC article.
-
P granule assembly and function in Caenorhabditis elegans germ cells.J Androl. 2010 Jan-Feb;31(1):53-60. doi: 10.2164/jandrol.109.008292. Epub 2009 Oct 29. J Androl. 2010. PMID: 19875490 Free PMC article. Review.
-
A mechanism for MEX-5-driven disassembly of PGL-3/RNA condensates in vitro.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2412218122. doi: 10.1073/pnas.2412218122. Epub 2025 May 12. Proc Natl Acad Sci U S A. 2025. PMID: 40354522 Free PMC article.
References
-
- Ahringer, J. (Editor), 2006. Reverse genetics, in WormBook, edited by The C. elegans Research Community. WormBook, http://www.wormbook.org.
-
- D'Agostino, I., C. Merritt, P. L. Chan, G. Seydoux and K. Subramaniam, 2006. Translational repression restricts expression of the C. elegans nanos homolog NOS-2 to the embryonic germline. Dev. Biol. 292: 244–252. - PubMed
-
- Jones, A. R., R. Francis and T. Schedl, 1996. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage-and sex-specific expression during Caenorhabditis elegans germline development. Dev. Biol. 180: 165–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

