Characterization of interactions between and among components of the meiotic silencing by unpaired DNA machinery in Neurospora crassa using bimolecular fluorescence complementation
- PMID: 18202398
- PMCID: PMC2206107
- DOI: 10.1534/genetics.107.079384
Characterization of interactions between and among components of the meiotic silencing by unpaired DNA machinery in Neurospora crassa using bimolecular fluorescence complementation
Abstract
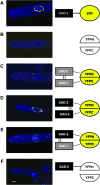
Bimolecular fluorescence complementation (BiFC) is based on the complementation between two nonfluorescent fragments of the yellow fluorescent protein (YFP) when they are united by interactions between proteins covalently linked to them. We have successfully applied BiFC in Neurospora crassa using two genes involved in meiotic silencing by unpaired DNA (MSUD) and observed macromolecular complex formation involving only SAD-1 proteins, only SAD-2 proteins, and mixtures of SAD-1 and SAD-2 proteins.
Figures

Similar articles
-
Novel proteins required for meiotic silencing by unpaired DNA and siRNA generation in Neurospora crassa.Genetics. 2013 May;194(1):91-100. doi: 10.1534/genetics.112.148999. Epub 2013 Mar 15. Genetics. 2013. PMID: 23502675 Free PMC article.
-
Complex formation of RNA silencing proteins in the perinuclear region of Neurospora crassa.Genetics. 2015 Apr;199(4):1017-21. doi: 10.1534/genetics.115.174623. Epub 2015 Feb 2. Genetics. 2015. PMID: 25644701 Free PMC article.
-
Fluorescent and bimolecular-fluorescent protein tagging of genes at their native loci in Neurospora crassa using specialized double-joint PCR plasmids.Fungal Genet Biol. 2011 Sep;48(9):866-73. doi: 10.1016/j.fgb.2011.05.002. Epub 2011 May 31. Fungal Genet Biol. 2011. PMID: 21664475
-
Sixteen Years of Meiotic Silencing by Unpaired DNA.Adv Genet. 2017;97:1-42. doi: 10.1016/bs.adgen.2016.11.001. Epub 2016 Dec 29. Adv Genet. 2017. PMID: 28838355 Review.
-
RNA silencing in fungi: mechanisms and applications.FEBS Lett. 2005 Oct 31;579(26):5950-7. doi: 10.1016/j.febslet.2005.08.016. Epub 2005 Aug 24. FEBS Lett. 2005. PMID: 16137680 Review.
Cited by
-
GPCR dimerization: Drug discovery aspects and targets in renin-angiotensin systems.FASEB Bioadv. 2025 Jan 31;7(3):e1486. doi: 10.1096/fba.2024-00180. eCollection 2025 Mar. FASEB Bioadv. 2025. PMID: 40060944 Free PMC article. Review.
-
RNA interference pathways in fungi: mechanisms and functions.Annu Rev Microbiol. 2012;66:305-23. doi: 10.1146/annurev-micro-092611-150138. Epub 2012 Jun 28. Annu Rev Microbiol. 2012. PMID: 22746336 Free PMC article. Review.
-
Diversity in genetic in vivo methods for protein-protein interaction studies: from the yeast two-hybrid system to the mammalian split-luciferase system.Microbiol Mol Biol Rev. 2012 Jun;76(2):331-82. doi: 10.1128/MMBR.05021-11. Microbiol Mol Biol Rev. 2012. PMID: 22688816 Free PMC article. Review.
-
An NCBP3-Domain Protein Mediates Meiotic Silencing by Unpaired DNA.G3 (Bethesda). 2020 Jun 1;10(6):1919-1927. doi: 10.1534/g3.120.401236. G3 (Bethesda). 2020. PMID: 32291289 Free PMC article.
-
The Nuclear Cap-Binding Complex Mediates Meiotic Silencing by Unpaired DNA.G3 (Bethesda). 2017 Apr 3;7(4):1149-1155. doi: 10.1534/g3.116.038679. G3 (Bethesda). 2017. PMID: 28179391 Free PMC article.
References
-
- Davis, R. H., and F. J. De Serres, 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 17A: 79–143.
-
- Ebbole, D., and M. S. Sachs, 1990. A rapid and simple method for isolation of Neurospora crassa homokaryons using microconidia. Fungal Genet. Newsl. 37: 17–18.
-
- Freitag, M., P. C. Hickey, N. B. Raju, E. U. Selker and N. D. Read, 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41: 897–910. - PubMed
-
- Hoff, B., and U. Kück, 2005. Use of bimolecular fluorescence complementation to demonstrate transcription factor interaction in nuclei of living cells from the filamentous fungus Acremonium chrysogenum. Curr. Genet. 47: 132–138. - PubMed
-
- Hu, C. D., Y. Chinenov and T. K. Kerppola, 2002. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell 9: 789–798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

