Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis
- PMID: 18202436
- PMCID: PMC2223845
- DOI: 10.1128/CMR.00019-07
Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis
Abstract
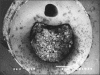
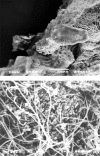
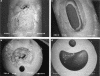
Catheter-associated urinary tract infections (CAUTIs) represent the most common type of nosocomial infection and are a major health concern due to the complications and frequent recurrence. These infections are often caused by Escherichia coli and Proteus mirabilis. Gram-negative bacterial species that cause CAUTIs express a number of virulence factors associated with adhesion, motility, biofilm formation, immunoavoidance, and nutrient acquisition as well as factors that cause damage to the host. These infections can be reduced by limiting catheter usage and ensuring that health care professionals correctly use closed-system Foley catheters. A number of novel approaches such as condom and suprapubic catheters, intermittent catheterization, new surfaces, catheters with antimicrobial agents, and probiotics have thus far met with limited success. While the diagnosis of symptomatic versus asymptomatic CAUTIs may be a contentious issue, it is generally agreed that once a catheterized patient is believed to have a symptomatic urinary tract infection, the catheter is removed if possible due to the high rate of relapse. Research focusing on the pathogenesis of CAUTIs will lead to a better understanding of the disease process and will subsequently lead to the development of new diagnosis, prevention, and treatment options.
Figures




References
-
- Abraham, S., J. Shin, and R. Malaviya. 2001. Type 1 fimbriated Escherichia coli-mast cell interactions in cystitis. J. Infect. Dis. 183(Suppl. 1):S51-S55. - PubMed
-
- Allison, C., L. Emody, N. Coleman, and C. Hughes. 1994. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169:1155-1158. - PubMed
-
- Allison, C., H. C. Lai, D. Gygi, and C. Hughes. 1993. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol. Microbiol. 8:53-60. - PubMed
-
- Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. - PubMed
-
- Altman, E., B. A. Harrison, R. K. Latta, K. K. Lee, J. F. Kelly, and P. Thibault. 2001. Galectin-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem. Cell Biol. 79:783-788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

