Identification of RPS14 as a 5q- syndrome gene by RNA interference screen
- PMID: 18202658
- PMCID: PMC3771855
- DOI: 10.1038/nature06494
Identification of RPS14 as a 5q- syndrome gene by RNA interference screen
Abstract
Somatic chromosomal deletions in cancer are thought to indicate the location of tumour suppressor genes, by which a complete loss of gene function occurs through biallelic deletion, point mutation or epigenetic silencing, thus fulfilling Knudson's two-hit hypothesis. In many recurrent deletions, however, such biallelic inactivation has not been found. One prominent example is the 5q- syndrome, a subtype of myelodysplastic syndrome characterized by a defect in erythroid differentiation. Here we describe an RNA-mediated interference (RNAi)-based approach to discovery of the 5q- disease gene. We found that partial loss of function of the ribosomal subunit protein RPS14 phenocopies the disease in normal haematopoietic progenitor cells, and also that forced expression of RPS14 rescues the disease phenotype in patient-derived bone marrow cells. In addition, we identified a block in the processing of pre-ribosomal RNA in RPS14-deficient cells that is functionally equivalent to the defect in Diamond-Blackfan anaemia, linking the molecular pathophysiology of the 5q- syndrome to a congenital syndrome causing bone marrow failure. These results indicate that the 5q- syndrome is caused by a defect in ribosomal protein function and suggest that RNAi screening is an effective strategy for identifying causal haploinsufficiency disease genes.
Figures
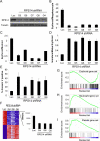
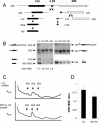

Comment in
-
Cancer: hay in a haystack.Nature. 2008 Jan 17;451(7176):252-3. doi: 10.1038/451252a. Nature. 2008. PMID: 18202630 No abstract available.
References
-
- Van den Berghe H, et al. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974;251(5474):437. - PubMed
-
- Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340(21):1649. - PubMed
-
- Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12(1):5. - PubMed
-
- List A, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

