Identification of cells initiating human melanomas
- PMID: 18202660
- PMCID: PMC3660705
- DOI: 10.1038/nature06489
Identification of cells initiating human melanomas
Abstract
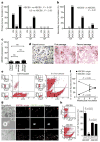
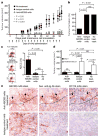
Tumour-initiating cells capable of self-renewal and differentiation, which are responsible for tumour growth, have been identified in human haematological malignancies and solid cancers. If such minority populations are associated with tumour progression in human patients, specific targeting of tumour-initiating cells could be a strategy to eradicate cancers currently resistant to systemic therapy. Here we identify a subpopulation enriched for human malignant-melanoma-initiating cells (MMIC) defined by expression of the chemoresistance mediator ABCB5 (refs 7, 8) and show that specific targeting of this tumorigenic minority population inhibits tumour growth. ABCB5+ tumour cells detected in human melanoma patients show a primitive molecular phenotype and correlate with clinical melanoma progression. In serial human-to-mouse xenotransplantation experiments, ABCB5+ melanoma cells possess greater tumorigenic capacity than ABCB5- bulk populations and re-establish clinical tumour heterogeneity. In vivo genetic lineage tracking demonstrates a specific capacity of ABCB5+ subpopulations for self-renewal and differentiation, because ABCB5+ cancer cells generate both ABCB5+ and ABCB5- progeny, whereas ABCB5- tumour populations give rise, at lower rates, exclusively to ABCB5- cells. In an initial proof-of-principle analysis, designed to test the hypothesis that MMIC are also required for growth of established tumours, systemic administration of a monoclonal antibody directed at ABCB5, shown to be capable of inducing antibody-dependent cell-mediated cytotoxicity in ABCB5+ MMIC, exerted tumour-inhibitory effects. Identification of tumour-initiating cells with enhanced abundance in more advanced disease but susceptibility to specific targeting through a defining chemoresistance determinant has important implications for cancer therapy.
Figures

References
-
- Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 1997;3:730–737. - PubMed
-
- Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. - PubMed
-
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

