Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice
- PMID: 18202746
- PMCID: PMC2200299
- DOI: 10.1172/JCI32525
Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice
Abstract
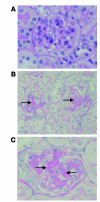
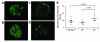
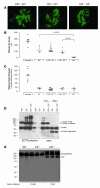
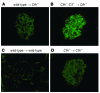
The inflammatory kidney disease membranoproliferative glomerulonephritis type II (MPGN2) is associated with dysregulation of the alternative pathway of complement activation. MPGN2 is characterized by the presence of complement C3 along the glomerular basement membrane (GBM). Spontaneous activation of C3 through the alternative pathway is regulated by 2 plasma proteins, factor H and factor I. Deficiency of either of these regulators results in uncontrolled C3 activation, although the breakdown of activated C3 is dependent on factor I. Deficiency of factor H, but not factor I, is associated with MPGN2 in humans, pigs, and mice. To explain this discordance, mice with single or combined deficiencies of these factors were studied. MPGN2 did not develop in mice with combined factor H and I deficiency or in mice deficient in factor I alone. However, administration of a source of factor I to mice with combined factor H and factor I deficiency triggered both activated C3 fragments in plasma and GBM C3 deposition. Mouse renal transplant studies demonstrated that C3 deposited along the GBM was derived from plasma. Together, these findings provide what we believe to be the first evidence that factor I-mediated generation of activated C3 fragments in the circulation is a critical determinant for the development of MPGN2 associated with factor H deficiency.
Figures



References
-
- Pickering M.C., Botto M., Taylor P.R., Lachmann P.J., Walport M.J. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 2000;76:227–324. - PubMed
-
- Liszewski M.K., Farries T.C., Lublin D.M., Rooney I.A., Atkinson J.P. Control of the complement system. Adv. Immunol. 1996;61:201–283. - PubMed
-
- Lachmann P.J., Nicol P. Reaction mechanism of the alternative pathway of complement fixation. Lancet. 1973;1:465–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

