Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease
- PMID: 18203133
- PMCID: PMC2423340
- DOI: 10.1002/mnfr.200700412
Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease
Abstract
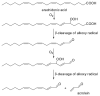
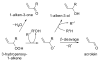
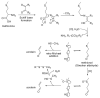
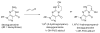
Acrolein (2-propenal) is ubiquitously present in (cooked) foods and in the environment. It is formed from carbohydrates, vegetable oils and animal fats, amino acids during heating of foods, and by combustion of petroleum fuels and biodiesel. Chemical reactions responsible for release of acrolein include heat-induced dehydration of glycerol, retro-aldol cleavage of dehydrated carbohydrates, lipid peroxidation of polyunsaturated fatty acids, and Strecker degradation of methionine and threonine. Smoking of tobacco products equals or exceeds the total human exposure to acrolein from all other sources. The main endogenous sources of acrolein are myeloperoxidase-mediated degradation of threonine and amine oxidase-mediated degradation of spermine and spermidine, which may constitute a significant source of acrolein in situations of oxidative stress and inflammation. Acrolein is metabolized by conjugation with glutathione and excreted in the urine as mercapturic acid metabolites. Acrolein forms Michael adducts with ascorbic acid in vitro, but the biological relevance of this reaction is not clear. The biological effects of acrolein are a consequence of its reactivity towards biological nucleophiles such as guanine in DNA and cysteine, lysine, histidine, and arginine residues in critical regions of nuclear factors, proteases, and other proteins. Acrolein adduction disrupts the function of these biomacromolecules which may result in mutations, altered gene transcription, and modulation of apoptosis.
Figures







References
-
- Chevreul E. Rechereches chimiques sur les corps gras d’origine animale. Imprimerie Nationale; Paris: 1823.
-
- Berzelius J. Lehrbuch der Chemie. Dresden and Leipzig; 1839.
-
- Redtenbacher J. Ueber die Zerlegungsprodukte des Glycerinoxydes durch trockene Destillation. Ann. Chem. Pharm. 1843;47:113–148.
-
- Roscoe H, Schorlemmer C. A Treatise on Chemistry. Volume III: The Chemistry of the Hydrocarbons and Their Derivatives. D. Appleton and Company; New York: 1884.
-
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 1991;11:81–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

