Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection
- PMID: 18203140
- PMCID: PMC2955512
- DOI: 10.1002/eji.200737268
Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection
Abstract
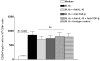
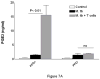
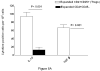
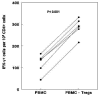
We evaluated the role of regulatory T cells (CD4(+) CD25(+) Foxp3(+) cells, Tregs) in human Mycobacterium tuberculosis infection. Tregs were expanded in response to M. tuberculosis in healthy tuberculin reactors, but not in tuberculin-negative individuals. The M. tuberculosis mannose-capped lipoarabinomannan (ManLAM) resulted in regulatory T cell expansion, whereas the M. tuberculosis 19-kDa protein and heat shock protein 65 had no effect. Anti-IL-10 and anti-TGF-beta alone or in combination, did not reduce expansion of Tregs. In contrast, the cyclooxygenase enzyme-2 inhibitor NS398 significantly inhibited expansion of Tregs, indicating that prostaglandin E2 (PGE2) contributes to Treg expansion. Monocytes produced PGE2 upon culturing with heat-killed M. tuberculosis or ManLAM, and T cells from healthy tuberculin reactors enhanced PGE2 production by monocytes. Expanded Tregs produced significant amounts of TGF-beta and IL-10 and depletion of Tregs from PBMC of these individuals increased the frequency of M. tuberculosis-responsive CD4(+) IFN-gamma cells. Culturing M. tuberculosis-expanded Tregs with autologous CD8(+) cells decreased the frequency of IFN-gamma(+)cells. Freshly isolated PBMC from tuberculosis patients had increased percentages of Tregs, compared to healthy tuberculin reactors. These findings demonstrate that Tregs expand in response to M. tuberculosis through mechanisms that depend on ManLAM and PGE2.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





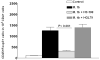




Similar articles
-
Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation With Mycobacterium tuberculosis.J Infect Dis. 2011 May 1;203(9):1256-63. doi: 10.1093/infdis/jir011. Epub 2011 Mar 7. J Infect Dis. 2011. PMID: 21383382 Free PMC article.
-
CD4+ CD25(high) Foxp3+ regulatory T cells downregulate human Vdelta2+ T-lymphocyte function triggered by anti-CD3 or phosphoantigen.Immunology. 2009 Jul;127(3):398-407. doi: 10.1111/j.1365-2567.2008.02982.x. Epub 2008 Nov 14. Immunology. 2009. PMID: 19019089 Free PMC article.
-
NK cells lyse T regulatory cells that expand in response to an intracellular pathogen.J Immunol. 2008 Feb 1;180(3):1729-36. doi: 10.4049/jimmunol.180.3.1729. J Immunol. 2008. PMID: 18209070
-
NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen.J Immunol. 2004 Jan 1;172(1):130-7. doi: 10.4049/jimmunol.172.1.130. J Immunol. 2004. PMID: 14688318
-
Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jun 1;76(4):fty026. doi: 10.1093/femspd/fty026. Pathog Dis. 2018. PMID: 29722821 Free PMC article. Review.
Cited by
-
The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health.Curr Environ Health Rep. 2016 Sep;3(3):270-86. doi: 10.1007/s40572-016-0100-5. Curr Environ Health Rep. 2016. PMID: 27436048 Free PMC article. Review.
-
Dynamics of adrenal steroids are related to variations in Th1 and Treg populations during Mycobacterium tuberculosis infection in HIV positive persons.PLoS One. 2012;7(3):e33061. doi: 10.1371/journal.pone.0033061. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22431997 Free PMC article.
-
A Mouse Model of Chronic West Nile Virus Disease.PLoS Pathog. 2016 Nov 2;12(11):e1005996. doi: 10.1371/journal.ppat.1005996. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27806117 Free PMC article.
-
Macrophage-microbe interaction: lessons learned from the pathogen Mycobacterium tuberculosis.Semin Immunopathol. 2018 Nov;40(6):577-591. doi: 10.1007/s00281-018-0710-0. Epub 2018 Oct 10. Semin Immunopathol. 2018. PMID: 30306257 Review.
-
IL-17 and IL-22 production in HIV+ individuals with latent and active tuberculosis.BMC Infect Dis. 2018 Jul 11;18(1):321. doi: 10.1186/s12879-018-3236-0. BMC Infect Dis. 2018. PMID: 29996789 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

