Clinical applications of perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics
- PMID: 18203420
- PMCID: PMC2676820
Clinical applications of perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics
Abstract
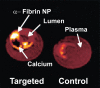
Molecular imaging is a novel tool that has allowed non-invasive diagnostic imaging to transition from gross anatomical description to identification of specific tissue epitopes and observation of biological processes at the cellular level. This technique has been confined to the field of nuclear imaging; however, recent advances in nanotechnology have extended this research to include ultrasound (US) and magnetic resonance (MR) imaging. The exploitation of nanotechnology for MR and US molecular imaging has generated several candidate contrast agents. One multimodality platform, targeted perfluorocarbon (PFC) nanoparticles, is useful for noninvasive detection with US and MR, targeted drug delivery, and quantification.
Figures








References
-
- Ambrose JA, Tannenbaum MA, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. Journal of the American College of Cardiology. 1988;12:56–62. - PubMed
-
- Anderson SA, Rader RK, et al. Magnetic resonance contrast enhancement of neovasculature with alpha(v)beta(3)-targeted nanoparticles. Magnetic Resonance in Medicine. 2000;44:433–9. - PubMed
-
- Bishop GG, McPherson JA, et al. Selective alpha(v)beta(3)-receptor blockade reduces macrophage infiltration and restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation. 2001;103:1906–11. - PubMed
-
- Brooks PC, Clark RA, et al. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

