Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers
- PMID: 18203429
- PMCID: PMC2676814
Biomimetic and bioactive nanofibrous scaffolds from electrospun composite nanofibers
Abstract
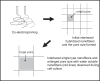
Electrospinning is an enabling technology that can architecturally (in terms of geometry, morphology or topography) and biochemically fabricate engineered cellular scaffolds that mimic the native extracellular matrix (ECM). This is especially important and forms one of the essential paradigms in the area of tissue engineering. While biomimesis of the physical dimensions of native ECM's major constituents (eg, collagen) is no longer a fabrication-related challenge in tissue engineering research, conveying bioactivity to electrospun nanofibrous structures will determine the efficiency of utilizing electrospun nanofibers for regenerating biologically functional tissues. This can certainly be achieved through developing composite nanofibers. This article gives a brief overview on the current development and application status of employing electrospun composite nanofibers for constructing biomimetic and bioactive tissue scaffolds. Considering that composites consist of at least two material components and phases, this review details three different configurations of nanofibrous composite structures by using hybridizing basic binary material systems as example. These are components blended composite nanofiber, core-shell structured composite nanofiber, and nanofibrous mingled structure.
Figures






Similar articles
-
Electrospun bioscaffolds that mimic the topology of extracellular matrix.Nanomedicine. 2006 Mar;2(1):37-41. doi: 10.1016/j.nano.2006.01.002. Nanomedicine. 2006. PMID: 17292114
-
Electrospun nanostructured scaffolds for bone tissue engineering.Acta Biomater. 2009 Oct;5(8):2884-93. doi: 10.1016/j.actbio.2009.05.007. Epub 2009 May 15. Acta Biomater. 2009. PMID: 19447211
-
Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering.Tissue Eng. 2004 Jul-Aug;10(7-8):1160-8. doi: 10.1089/ten.2004.10.1160. Tissue Eng. 2004. PMID: 15363172
-
Electrospun nanofiber scaffolds: engineering soft tissues.Biomed Mater. 2008 Sep;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. Epub 2008 Aug 8. Biomed Mater. 2008. PMID: 18689924 Review.
-
Biodegradable nanomats produced by electrospinning: expanding multifunctionality and potential for tissue engineering.J Nanosci Nanotechnol. 2006 Sep-Oct;6(9-10):2693-711. doi: 10.1166/jnn.2006.485. J Nanosci Nanotechnol. 2006. PMID: 17048476 Review.
Cited by
-
Engineering collagenous analogs of connective tissue extracellular matrix.Front Bioeng Biotechnol. 2022 Oct 14;10:925838. doi: 10.3389/fbioe.2022.925838. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36312546 Free PMC article.
-
Microfabricated substrates as a tool to study cell mechanotransduction.Med Biol Eng Comput. 2010 Oct;48(10):965-76. doi: 10.1007/s11517-010-0619-9. Epub 2010 Apr 28. Med Biol Eng Comput. 2010. PMID: 20424924 Review.
-
Regenerative nanomedicines: an emerging investment prospective?J R Soc Interface. 2010 Dec 6;7 Suppl 6(Suppl 6):S783-7. doi: 10.1098/rsif.2010.0351.focus. Epub 2010 Sep 8. J R Soc Interface. 2010. PMID: 20826478 Free PMC article. Review.
-
Dual micelles-loaded gelatin nanofibers and their application in lipopolysaccharide-induced periodontal disease.Int J Nanomedicine. 2019 Feb 4;14:963-976. doi: 10.2147/IJN.S182073. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30787610 Free PMC article.
-
Microablation of collagen-based substrates for soft tissue engineering.Biomed Mater. 2014 Feb;9(1):011002. doi: 10.1088/1748-6041/9/1/011002. Epub 2014 Jan 23. Biomed Mater. 2014. PMID: 24457193 Free PMC article.
References
-
- Badami AS, Kreke MR, et al. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. - PubMed
-
- Baldwin SP, Mark Saltzman W. Materials for protein delivery in tissue engineering. Advanced Drug Delivery Reviews. 1998;33:71–86. - PubMed
-
- Bhattarai SR, Bhattarai N, et al. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials. 2004;25:2595–602. - PubMed
-
- Bradt J-H, Mertig M, et al. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem Mater. 1999;11:2694–701.
-
- Buck M. Trifluoroethanol and colleagues: cosolvents come of age. Recent studies with peptides and proteins. Quarterly Reviews of Biophysics. 1998;31:297–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

