Medicinal applications of fullerenes
- PMID: 18203430
- PMCID: PMC2676811
Medicinal applications of fullerenes
Abstract
Fullerenes have attracted considerable attention in different fields of science since their discovery in 1985. Investigations of physical, chemical and biological properties of fullerenes have yielded promising information. It is inferred that size, hydrophobicity, three-dimensionality and electronic configurations make them an appealing subject in medicinal chemistry. Their unique carbon cage structure coupled with immense scope for derivatization make them a potential therapeutic agent. The study of biological applications has attracted increasing attention despite the low solubility of carbon spheres in physiological media. The fullerene family, and especially C60, has appealing photo, electrochemical and physical properties, which can be exploited in various medical fields. Fullerene is able to fit inside the hydrophobic cavity of HIV proteases, inhibiting the access of substrates to the catalytic site of enzyme. It can be used as radical scavenger and antioxidant. At the same time, if exposed to light, fullerene can produce singlet oxygen in high quantum yields. This action, together with direct electron transfer from excited state of fullerene and DNA bases, can be used to cleave DNA. In addition, fullerenes have been used as a carrier for gene and drug delivery systems. Also they are used for serum protein profiling as MELDI material for biomarker discovery. In this review we report the aspects of medicinal applications of fullerenes.
Figures


 ) Copyright © 1995. Reproduced with permission from Rywkin S, Ben-Hur E, Reid ME, et al. 1995. Selective protection against IgG binding to red cells treated with phthalocyanines and red light for virus inactivation. Transfusion, 35:414–20.
) Copyright © 1995. Reproduced with permission from Rywkin S, Ben-Hur E, Reid ME, et al. 1995. Selective protection against IgG binding to red cells treated with phthalocyanines and red light for virus inactivation. Transfusion, 35:414–20.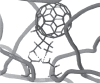

Similar articles
-
Fullerene derivatives: an attractive tool for biological applications.Eur J Med Chem. 2003 Nov-Dec;38(11-12):913-23. doi: 10.1016/j.ejmech.2003.09.005. Eur J Med Chem. 2003. PMID: 14642323 Review.
-
Carbon nanomaterials: Biologically active fullerene derivatives.Srp Arh Celok Lek. 2016 Mar-Apr;144(3-4):222-31. Srp Arh Celok Lek. 2016. PMID: 27483572 Review.
-
C60-fullerenes as Drug Delivery Carriers for Anticancer Agents: Promises and Hurdles.Pharm Nanotechnol. 2017;5(3):169-179. doi: 10.2174/2211738505666170301142232. Pharm Nanotechnol. 2017. PMID: 29361902 Review.
-
Fullerenes for applications in biology and medicine.Curr Med Chem. 2011;18(14):2045-59. doi: 10.2174/092986711795656225. Curr Med Chem. 2011. PMID: 21517770
-
An Update Report on the Biosafety and Potential Toxicity of Fullerene-Based Nanomaterials toward Aquatic Animals.Oxid Med Cell Longev. 2021 Jul 17;2021:7995223. doi: 10.1155/2021/7995223. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34336114 Free PMC article. Review.
Cited by
-
Adsorption of drugs on B12N12 and Al12N12 nanocages.RSC Adv. 2024 Oct 8;14(43):31756-31767. doi: 10.1039/d4ra05586a. eCollection 2024 Oct 1. RSC Adv. 2024. PMID: 39380648 Free PMC article.
-
Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives.Nanomicro Lett. 2020 Feb 4;12(1):45. doi: 10.1007/s40820-020-0383-9. Nanomicro Lett. 2020. PMID: 34138283 Free PMC article. Review.
-
Influence of fullerene (C60) on soil bacterial communities: aqueous aggregate size and solvent co-introduction effects.Sci Rep. 2016 Jun 16;6:28069. doi: 10.1038/srep28069. Sci Rep. 2016. PMID: 27306076 Free PMC article.
-
Combined Action of Hyper-Harmonized Hydroxylated Fullerene Water Complex and Hyperpolarized Light Leads to Melanoma Cell Reprogramming In Vitro.Nanomaterials (Basel). 2022 Apr 13;12(8):1331. doi: 10.3390/nano12081331. Nanomaterials (Basel). 2022. PMID: 35458039 Free PMC article.
-
Facile and Green Synthesis of Novel Fluorescent Carbon Quantum Dots and Their Silver Heterostructure: An In Vitro Anticancer Activity and Imaging on Colorectal Carcinoma.ACS Omega. 2023 Jan 27;8(5):4566-4577. doi: 10.1021/acsomega.2c04964. eCollection 2023 Feb 7. ACS Omega. 2023. PMID: 36777585 Free PMC article.
References
-
- An YZ, Chen CHB, Anderson JL, et al. Sequence-specific modification of guanosine in DNA by a C60-linked deoxyoligonucleo-tide: evidence for a non-singlet oxygen mechanism. Tetrahedron. 1996;52:5179–89.
-
- Azzam T, Domb AJ. Current developments in gene transfection agents. Curr Drug Deliv. 2004;1:165–93. - PubMed
-
- Bensasson RV, Bienvenue E, Dellinger M, et al. C60 in model biological systems. A visible-UV absorption study of solvent-dependent parameters and solute aggregation. J Phys Chem. 1994;98:3492–500.
-
- Bianco A, Maggini M, Scorrano G, et al. Synthesis, chiroptical properties, and configurational assignment of fulleroproline derivatives and peptides. J Am Chem Soc. 1996;118:4072–80.
-
- Bisaglia M, Natalini B, Pellicciari R, et al. C3-fullero-tris-methanodicarboxylic acid protects cerebellar granule cells from apoptosis. J Neurochem. 2000;74:1197–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

