Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization, and pulmonary pharmacokinetics
- PMID: 18203434
- PMCID: PMC2676822
Nano-liposomal dry powder inhaler of tacrolimus: preparation, characterization, and pulmonary pharmacokinetics
Abstract
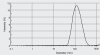
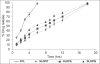
The studies were undertaken to evaluate feasibility of pulmonary delivery of liposomaly encapsulated tacrolimus dry powder inhaler for prolonged drug retention in lungs as rescue therapy to prevent refractory rejection of lungs after transplantation. Tacrolimus encapsulated liposomes were prepared by thin film evaporation technique and liposomal dispersion was passed through high pressure homogenizer. Tacrolimus nano-liposomes (NLs) were separated by centrifugation and characterized. NLs were dispersed in phosphate buffer saline (PBS) pH 7.4 containing different additives like lactose, sucrose, and trehalose, and L-leucine as antiadherent. The dispersion was spray dried and spray dried powders were characterized. In vitro and in vivo pulmonary deposition was performed using Andersen Cascade Impactor and intratracheal instillation in rats respectively. NLs were found to have average size of 140 nm, 96% +/- 1.5% drug entrapment, and zeta potential of 1.107 mV. Trehalose based formulation was found to have low density, good flowability, particle size of 9.46 +/- 0.8 microm, maximum fine particle fraction (FPF) of 71.1 +/- 2.5%, mean mass aerodynamic diameter (MMAD) 2.2 +/- 0.1 microm, and geometric standard deviation (GSD) 1.7 +/- 0.2. Developed formulations were found to have in vitro prolonged drug release up to 18 hours, following Higuchi's Controlled Release model. In vivo studies revealed maximal residence of tacrolimus within lungs of 24 hours, suggesting slow clearance from the lungs. The investigation provides a practical approach for direct delivery of tacrolimus encapsulated in NLs for controlled and prolonged retention at the site of action. It may play a promising role as rescue therapy in reducing the risk of acute rejection and chronic rejection.
Figures


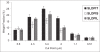
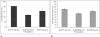
Similar articles
-
Development of spray dried liposomal dry powder inhaler of Dapsone.AAPS PharmSciTech. 2008;9(1):47-53. doi: 10.1208/s12249-007-9024-6. Epub 2008 Jan 9. AAPS PharmSciTech. 2008. PMID: 18446460 Free PMC article.
-
Nano-liposomal dry powder inhaler of Amiloride Hydrochloride.J Nanosci Nanotechnol. 2006 Sep-Oct;6(9-10):3001-9. doi: 10.1166/jnn.2006.405. J Nanosci Nanotechnol. 2006. PMID: 17048511
-
Lung delivery of nanoliposomal salbutamol sulfate dry powder inhalation for facilitated asthma therapy.J Liposome Res. 2019 Dec;29(4):332-342. doi: 10.1080/08982104.2018.1531022. Epub 2019 May 23. J Liposome Res. 2019. PMID: 30296863
-
In vitro and in vivo performance of dry powder inhalation formulations: comparison of particles prepared by thin film freezing and micronization.AAPS PharmSciTech. 2014 Aug;15(4):981-93. doi: 10.1208/s12249-014-0126-7. Epub 2014 May 14. AAPS PharmSciTech. 2014. PMID: 24824172 Free PMC article.
-
Recent advances in liposomal dry powder formulations: preparation and evaluation.Expert Opin Drug Deliv. 2009 Jan;6(1):71-89. doi: 10.1517/17425240802652309. Expert Opin Drug Deliv. 2009. PMID: 19236209 Review.
Cited by
-
Dry Powder for Pulmonary Delivery: A Comprehensive Review.Pharmaceutics. 2020 Dec 28;13(1):31. doi: 10.3390/pharmaceutics13010031. Pharmaceutics. 2020. PMID: 33379136 Free PMC article. Review.
-
Formulation strategies for drug delivery of tacrolimus: An overview.Int J Pharm Investig. 2012 Oct;2(4):169-75. doi: 10.4103/2230-973X.106981. Int J Pharm Investig. 2012. PMID: 23580932 Free PMC article.
-
STAT6 siRNA matrix-loaded gelatin nanocarriers: formulation, characterization, and ex vivo proof of concept using adenocarcinoma cells.Biomed Res Int. 2013;2013:858946. doi: 10.1155/2013/858946. Epub 2013 Sep 26. Biomed Res Int. 2013. PMID: 24191252 Free PMC article.
-
Inhalation Dosage Forms: A Focus on Dry Powder Inhalers and Their Advancements.Pharmaceuticals (Basel). 2023 Nov 28;16(12):1658. doi: 10.3390/ph16121658. Pharmaceuticals (Basel). 2023. PMID: 38139785 Free PMC article. Review.
-
Development of spray dried liposomal dry powder inhaler of Dapsone.AAPS PharmSciTech. 2008;9(1):47-53. doi: 10.1208/s12249-007-9024-6. Epub 2008 Jan 9. AAPS PharmSciTech. 2008. PMID: 18446460 Free PMC article.
References
-
- Alemdar AY, Sadi D, McAlister VC, et al. Liposomal formulations of tacrolimus and rapamycin increase graft survival and fiber outgrowth of dopaminergic grafts. Cell Transplant. 2004;13:263–71. - PubMed
-
- Arima H, Yunomae K, Miyake K, et al. Comparative studies of the enhancing effects of cyclodextrins on the solubility and oral bioavailability of tacrolimus in rats. J Pharma Sci. 2001;90:690–701. - PubMed
-
- Arppe J, Vidgren M, Waldrep JC. Pulmonary pharmacokinetics of cyclosporin A liposomes. Int J of Pharm. 1998;161:205–14.
-
- Boehler A, Estenne M. Obliterative bronchiolitis after lung transplantation. Curr Opin Pulm Med. 2000;6:133–9. - PubMed
-
- Bosquillon C, Rouxhet PG, TLimou F, et al. Aerosolization properties, surface composition and physical state of spray-dried protein powders. J Control Rel. 2004;99:357–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

