Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo
- PMID: 18203438
- PMCID: PMC2676807
Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo
Abstract
Protein cage nanoparticles have the potential to serve as multifunctional cell targeted, imaging and therapeutic platforms for broad applications in medicine. However, before they find applications in medicine, their biocompatibility in vivo needs to be demonstrated. We provide here baseline biodistribution information of two different spherical protein cage nanoplatforms, the 28 nm viral Cowpea chlorotic mottle virus (CCMV) and the 12 nm heat shock protein (Hsp) cage. In naive and immunized mice both nanoplatforms show similar broad distribution and movement throughout most tissues and organs, rapid excretion, the absence of long-term persistence within mice tissue and organs, and no overt toxicity after a single injection. These results suggest that protein cage based nanoparticles may serve as safe, biocompatible, nanoplatforms for applications in medicine.
Figures
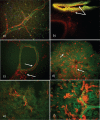
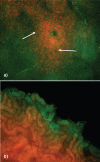
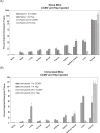
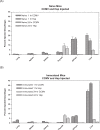
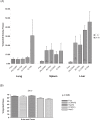
Similar articles
-
Biodistribution, pharmacokinetics, and toxicity of dendrimer-coated iron oxide nanoparticles in BALB/c mice.Int J Nanomedicine. 2018 Mar 13;13:1483-1493. doi: 10.2147/IJN.S157293. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29559777 Free PMC article.
-
Significant effect of size on the in vivo biodistribution of gold composite nanodevices in mouse tumor models.Nanomedicine. 2007 Dec;3(4):281-96. doi: 10.1016/j.nano.2007.09.001. Epub 2007 Oct 24. Nanomedicine. 2007. PMID: 17962085
-
Control of the in vivo biodistribution of hybrid nanoparticles with different poly(ethylene glycol) coatings.Small. 2009 Nov;5(22):2565-75. doi: 10.1002/smll.200900563. Small. 2009. PMID: 19768700
-
Enhanced detection with spectral imaging fluorescence microscopy reveals tissue- and cell-type-specific compartmentalization of surface-modified polystyrene nanoparticles.J Nanobiotechnology. 2016 Jul 7;14(1):55. doi: 10.1186/s12951-016-0210-0. J Nanobiotechnology. 2016. PMID: 27388915 Free PMC article.
-
In vivo biodistribution of nanoparticles.Nanomedicine (Lond). 2011 Jul;6(5):815-35. doi: 10.2217/nnm.11.79. Nanomedicine (Lond). 2011. PMID: 21793674 Review.
Cited by
-
Infusion of imaging and therapeutic molecules into the plant virus-based carrier cowpea mosaic virus: cargo-loading and delivery.J Control Release. 2013 Dec 10;172(2):568-78. doi: 10.1016/j.jconrel.2013.04.023. Epub 2013 May 9. J Control Release. 2013. PMID: 23665254 Free PMC article.
-
Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology.Adv Virus Res. 2017;97:1-60. doi: 10.1016/bs.aivir.2016.09.002. Epub 2016 Nov 8. Adv Virus Res. 2017. PMID: 28057256 Free PMC article. Review.
-
Synthetic plant virology for nanobiotechnology and nanomedicine.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 Jul;9(4):e1447. doi: 10.1002/wnan.1447. Epub 2017 Jan 11. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017. PMID: 28078770 Free PMC article. Review.
-
CD11c⁺ cells primed with unrelated antigens facilitate an accelerated immune response to influenza virus in mice.Eur J Immunol. 2014 Feb;44(2):397-408. doi: 10.1002/eji.201343587. Epub 2014 Jan 13. Eur J Immunol. 2014. PMID: 24222381 Free PMC article.
-
Exploitation of viral properties for intracellular delivery.J Pept Sci. 2014 Jul;20(7):468-78. doi: 10.1002/psc.2649. Epub 2014 May 30. J Pept Sci. 2014. PMID: 24889153 Free PMC article. Review.
References
-
- Allen M, Willits D, Mosolf J, et al. Protein cage constrained synthesis of ferrimagnetic iron oxide nanoparticles. Advanced Materials. 2002;14:1562.
-
- Allen MA, Leipold LO, Bulte JWM, et al. Paramagnetic viral nanoparticles as potential high-relaxivity MRI contrast agents. Magn Reson Med. 2005;54:807–12. - PubMed
-
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. - PubMed
-
- Bancroft JB, Hiebert E. Formation of an infectious nucleoprotein from protein and nucleic acid isolated from a small spherical virus. Virology. 1967;32:354–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

