Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles
- PMID: 18203445
- PMCID: PMC2676812
Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles
Abstract
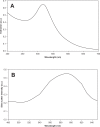
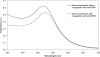
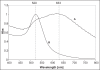
The high mortality rate in cancer such as oral squamous cell carcinoma is commonly attributed to the difficulties in detecting the disease at an early treatable stage. In this study, we exploited the ability of gold nanoparticles to undergo coupled surface plasmon resonance and set up strong electric fields when closely-spaced to improve the molecular contrast signal in reflectance-based imaging and also to enhance the Raman signal of bioanalytes in cancer. Colloidal gold nanoparticles were synthesized and conjugated to anti-epidermal growth factor receptor (EGFR) for imaging. A self-assembled surface enhanced Raman scattering (SERS)-active gold nanoparticle monolayer film was also developed as a biosensing surface using a simple drop-dry approach. We have shown that gold nanoparticles could elicit an optical contrast to discriminate between cancerous and normal cells and their conjugation with antibodies allowed them to map the expression of relevant biomarkers for molecular imaging under confocal reflectance microscopy. We have also shown that the SERS spectra of saliva from the closely-packed gold nanoparticles films was differentiable between those acquired from normal individuals and oral cancer patients, thus showing promise of a simple SERS-based saliva assay for early diagnosis of oral cancer.
Figures





Similar articles
-
Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer.Nano Lett. 2005 May;5(5):829-34. doi: 10.1021/nl050074e. Nano Lett. 2005. PMID: 15884879
-
Nanoparticle-based assay for detection of S100P mRNA using surface-enhanced Raman spectroscopy.J Biomed Opt. 2019 May;24(5):1-9. doi: 10.1117/1.JBO.24.5.055001. J Biomed Opt. 2019. PMID: 31066245 Free PMC article.
-
The potential use of the enhanced nonlinear properties of gold nanospheres in photothermal cancer therapy.Lasers Surg Med. 2007 Oct;39(9):747-53. doi: 10.1002/lsm.20577. Lasers Surg Med. 2007. PMID: 17960762
-
Surface-enhanced Raman scattering: realization of localized surface plasmon resonance using unique substrates and methods.Anal Bioanal Chem. 2009 Aug;394(7):1747-60. doi: 10.1007/s00216-009-2762-4. Epub 2009 Apr 22. Anal Bioanal Chem. 2009. PMID: 19384546 Review.
-
Role of Salivary Biomarkers in Oral Cancer Detection.Adv Clin Chem. 2018;86:23-70. doi: 10.1016/bs.acc.2018.05.002. Epub 2018 Jul 23. Adv Clin Chem. 2018. PMID: 30144841 Review.
Cited by
-
Multimodal in vivo imaging of oral cancer using fluorescence lifetime, photoacoustic and ultrasound techniques.Biomed Opt Express. 2013 Aug 26;4(9):1724-41. doi: 10.1364/BOE.4.001724. eCollection 2013. Biomed Opt Express. 2013. PMID: 24049693 Free PMC article.
-
Nanoparticles for Improving Cancer Diagnosis.Mater Sci Eng R Rep. 2013 Mar;74(3):35-69. doi: 10.1016/j.mser.2013.03.001. Mater Sci Eng R Rep. 2013. PMID: 24068857 Free PMC article.
-
Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors.Sensors (Basel). 2021 Feb 10;21(4):1253. doi: 10.3390/s21041253. Sensors (Basel). 2021. PMID: 33578726 Free PMC article. Review.
-
SERS properties of different sized and shaped gold nanoparticles biosynthesized under different environmental conditions by Neurospora crassa extract.PLoS One. 2013 Oct 9;8(10):e77486. doi: 10.1371/journal.pone.0077486. eCollection 2013. PLoS One. 2013. PMID: 24130891 Free PMC article.
-
Functionalized Gold Nanoparticles and Their Biomedical Applications.Nanomaterials (Basel). 2011 Jun 14;1(1):31-63. doi: 10.3390/nano1010031. Nanomaterials (Basel). 2011. PMID: 28348279 Free PMC article. Review.
References
-
- Abrams MJ, Murrer BA. Metal compounds in therapy and diagnosis. Science. 1993;261:725–30. - PubMed
-
- Bohren CF, Huffman DR. Absorption and Scattering of Light by Small Particles. New York: John Wiley and Sons; 1983.
-
- Chen YC, Li TY, Tsai MF. Analysis of the saliva from patients with oral cancer by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:364–9. - PubMed
-
- Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7. - PubMed
-
- Eghtedari MA, Copland JA, Popov YL, et al. Bioconjugated gold nanoparticles as a contrast agent for optoacoustic detection of small tumors. Proc SPIE. 2003;4960:76–85.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

