Do cross-modal projections always result in multisensory integration?
- PMID: 18203695
- PMCID: PMC2517110
- DOI: 10.1093/cercor/bhm230
Do cross-modal projections always result in multisensory integration?
Abstract
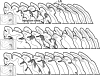
Convergence of afferents from different sensory modalities has generally been thought to produce bimodal (and trimodal) neurons (i.e., exhibit suprathreshold excitation to more than 1 sensory modality). Consequently, studies identifying cross-modal connections assume that such convergence results in bimodal (or trimodal) neurons that produce familiar forms of multisensory integration: response enhancement or depression. The present study questioned that assumption by anatomically identifying a projection from ferret auditory to visual cortex Area 21. However, electrophysiological recording within Area 21 not only failed to identify a single bimodal neuron but also familiar forms of multisensory integration were not observed either. Instead, a small proportion of neurons (9%; 27/296) showed subthreshold multisensory integration, in which visual responses were significantly modulated by auditory inputs. Such subthreshold multisensory effects were enhanced by gamma-aminobutyric acid antagonism, whereby a majority of neurons (87%; 20/23) now participated in a significant, multisensory population effect. Thus, multisensory convergence does not de facto result in bimodal (or trimodal) neurons or the traditional forms of multisensory integration. However, the fact that unimodal neurons exhibited a subthreshold form of multisensory integration not only affirms the relationship between convergence and integration but also expands our understanding of the functional repertoire of multisensory processing itself.
Figures






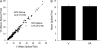

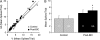
References
-
- Allman BL, Meredith MA. Multisensory processing in ‘unimodal’ neurons: cross-modal subthreshold auditory effects in cat extrastriate visual cortex. J Neurophysiol. 2007;98:545–549. - PubMed
-
- Barraclough NE, Xiao D, Baker CI, Oram MW, Perrett DI. Integration of visual and auditory information by superior temporal sulcus neurons responsive to the sight of actions. J Cog Neurosci. 2005;17:377–391. - PubMed
-
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nature Rev Neurosci. 2002;3:443–452. - PubMed
-
- Bell AH, Corneil BD, Munoz DP, Meredith MA. Engagement of visual fixation suppresses sensory responsiveness and multisensory integration in the primate superior colliculus. Eur J Neurosci. 2003;18:2867–2873. - PubMed

