The Trp-cage: optimizing the stability of a globular miniprotein
- PMID: 18203802
- PMCID: PMC3166533
- DOI: 10.1093/protein/gzm082
The Trp-cage: optimizing the stability of a globular miniprotein
Abstract
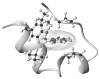
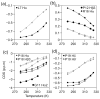
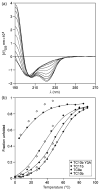
The Trp-cage, as the smallest miniprotein, remains the subject of numerous computational and experimental studies of protein folding dynamics and pathways. The original Trp-cage (NLYIQWLKDGGPSSGRPPPS, Tm = 42 degrees C) can be significantly stabilized by mutations; melting points as high as 64 degrees C are reported. In helical portions of the structure, each allowed replacement of Leu, Ile, Lys or Ser residues by Ala results in a 1.5 (+/-0.35) kJ/mol fold stabilization. No changes in structure or fluxionality of the core results upon stabilization. Contrary to the initial hypothesis, specific Pro/Trp interactions are not essential for core formation. The entropic advantage of Pro versus Ala (DeltaDeltaS(U) = 11 +/- 2 J/mol K) was measured at the solvent-exposed P17 site. Pro-Ala mutations at two of the three prolines (P12 and P18) that encage the indole ring result in less fold destabilization (2.3-3.4 kJ/mol). However, a P19A mutation reduces fold stability by 16 kJ/mol reflecting a favorable Y3/P19 interaction as well as Trp burial. The Y3/P19 hydrophobic staple interaction defines the folding motif as an 18-residue unit. Other stabilizing features that have been identified include a solvent-exposed Arg/Asp salt bridge (3.4-6 kJ/mol) and a buried H-bonded Ser side chain ( approximately 10 kJ/mol).
Figures




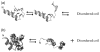
References
-
- Ahmed Z, Ilir AB, Mikhonin AV, Asher SA. J Am Chem Soc. 2005;127:10943–10950. - PubMed
-
- Andersen NH, Neidigh JW, Harris SM, Lee GM, Liu Z, Tong H. J Am Chem Soc. 1997;119:8547–8561.
-
- Andersen NH, Fesinmeyer RM, Neidigh JW, Barua B. In: The Trp-Cage: A Notably Stable Mini-Protein Fold in Peptides 2000. Martinez J, Fehrentz J-A, editors. EDK; Paris, France: 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

