Three-dimensional imaging of the highly bent architecture of Bdellovibrio bacteriovorus by using cryo-electron tomography
- PMID: 18203829
- PMCID: PMC2293184
- DOI: 10.1128/JB.01538-07
Three-dimensional imaging of the highly bent architecture of Bdellovibrio bacteriovorus by using cryo-electron tomography
Abstract
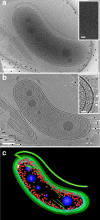
Bdellovibrio bacteriovorus cells are small deltaproteobacterial cells that feed on other gram-negative bacteria, including human pathogens. Using cryo-electron tomography, we demonstrated that B. bacteriovorus cells are capable of substantial flexibility and local deformation of the outer and inner membranes without loss of cell integrity. These shape changes can occur in less than 2 min, and analysis of the internal architecture of highly bent cells showed that the overall distribution of molecular machines and the nucleoid is similar to that in moderately bent cells. B. bacteriovorus cells appear to contain an extensive internal network of short and long filamentous structures. We propose that rearrangements of these structures, in combination with the unique properties of the cell envelope, may underlie the remarkable ability of B. bacteriovorus cells to find and enter bacterial prey.
Figures






References
-
- Bobyk, M. A., A. V. Afinogenova, M. V. Dudinskaya, V. A. Lambina, and I. S. Kulaev. 1980. Detection of polyphosphates and enzymes of polyphosphate metabolism in Bdellovibrio bacteriovorus. Zentralbl. Bakteriol. Naturwiss. 135461-466. - PubMed
-
- Briegel, A., D. P. Dias, Z. Li, R. B. Jensen, A. S. Frangakis, and G. J. Jensen. 2006. Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol. Microbiol. 625-14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

