Tumour endoproteases: the cutting edge of cancer drug delivery?
- PMID: 18204490
- PMCID: PMC2437906
- DOI: 10.1038/sj.bjp.0707657
Tumour endoproteases: the cutting edge of cancer drug delivery?
Abstract
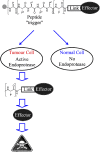
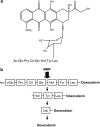
Despite progression in anticancer drug development and improvements in the clinical utilization of therapies, current treatment regimes are still dependent upon the use of systemic antiproliferative cytotoxic agents. Although these agents are unquestionably potent, their efficacy is limited by toxicity towards 'normal' cells and a lack of tumour selective targeting, resulting in a therapeutic index which is modest at best. Consequently, the development of more tumour selective cancer treatments, with better discrimination between tumour and normal cells is unequivocally an important goal for cancer drug discovery. One such strategy is to exploit the tumour phenotype as a mechanism for tumour-selective delivery of potent therapeutics. An exciting approach in this area is to develop anticancer therapeutics as prodrugs, which are non-toxic until activated by enzymes localized specifically in the tumour. Enzymes suitable for tumour-activated prodrug development must have increased activity in the tumour relative to non-diseased tissue and an ability to activate the prodrug to its active form. One class of enzyme satisfying these criteria are the tumour endoproteases, particularly the serine- and metallo-proteases. These proteolytic enzymes are essential for tumour angiogenesis, invasion and metastasis, the major defining features of malignancy. This review describes the concept behind development of tumour-endoprotease activated prodrugs and discusses the various studies to date that have demonstrated the huge potential of this approach for improvement of cancer therapy.
Figures

References
-
- Albright CF, Graciani N, Han W, Yue E, Stein R, Lai Z, et al. Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth better than doxorubicin with less toxicity. Mol Cancer Ther. 2005;4:751–760. - PubMed
-
- Atkinson JM, Falconer RA, Pennington CJ, Martin SW, Edwards DR, Patterson LH, et al. Membrane type 1-matrix metalloproteinase (MT1-MMP) targeted antitumour agents American Association for Cancer Research Annual Proceedings 2007a. Abstract: 2453
-
- Atkinson JM, Pennington CJ, Martin SW, Anikin VA, Mearns AJ, Loadman PM, et al. Membrane type matrix metalloproteinases (MMPs) show differential expression in non-small cell lung cancer (NSCLC) compared to normal lung: Correlation of MMP-14 mRNA expression and proteolytic activity. Eur J Cancer. 2007b;43:1764–1771. - PubMed
-
- Boddy AV, Yule SM. Metabolism and pharmacokinetics of oxazaphosphorines. Clin Pharmacokinet. 2000;38:291–304. - PubMed
-
- Borgono CA, Michael IP, Diamandis EP. Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res. 2004;2:257–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

