Defining the levels of secreted non-structural protein NS1 after West Nile virus infection in cell culture and mice
- PMID: 18205232
- PMCID: PMC2696118
- DOI: 10.1002/jmv.21091
Defining the levels of secreted non-structural protein NS1 after West Nile virus infection in cell culture and mice
Abstract
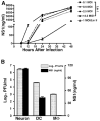
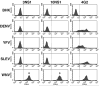
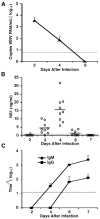
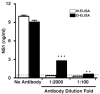
Infection with West Nile virus (WNV) causes a febrile illness that can progress to meningitis or encephalitis, primarily in humans that are immunocompromised or elderly. For successful treatment of WNV infection, accurate and timely diagnosis is essential. Previous studies have suggested that the flavivirus non-structural protein NS1, a highly conserved and secreted glycoprotein, is a candidate protein for rapid diagnosis. Herein, we developed a capture enzyme-linked immunosorbent assay (ELISA) to detect WNV NS1 using two anti-NS1 monoclonal antibodies (mAbs) that map to distinct sites on the protein. The capture ELISA efficiently detected as little as 0.5 ng/ml of soluble NS1 and exhibited no cross-reactivity for yellow fever, Dengue, and St. Louis encephalitis virus NS1. The capture ELISA reliably detected NS1 in plasma at day 3 after WNV infection, prior to the development of clinical signs of disease. As the time course of infection continued, the levels of detectable NS1 diminished, presumably because of interference by newly generated anti-NS1 antibodies. Indeed, treatment of plasma with a solution that dissociated NS1 immune complexes extended the window of detection. Overall, the NS1-based capture ELISA is a sensitive readout of infection and could be an important tool for diagnosis or screening small molecule inhibitors of WNV infection.
Figures


References
-
- Agrawal AG, Petersen LR. Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis. 2003;188:1–4. - PubMed
-
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–381. - PMC - PubMed
-
- Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating west nile virus infection in mice. J Infect Dis. 2003;188:5–12. - PubMed
-
- Brinton MA. The molecular biology of West Nile Virus: A new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. - PubMed
-
- Busch MP, Caglioti S, Robertson EF, McAuley JD, Tobler LH, Kamel H, Linnen JM, Shyamala V, Tomasulo P, Kleinman SH. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005a;353:460–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

