The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp
- PMID: 18205372
- PMCID: PMC2820876
- DOI: 10.1021/ol702952n
The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp
Abstract
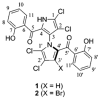
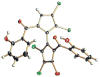
Cultivation of an obligate marine Streptomyces strain has furnished the marinopyrroles A and B, densely halogenated, axially chiral metabolites that contain an uncommon bispyrrole structure. X-ray analysis of marinopyrrole B showed that the natural product exists as an atropo-enantiomer with the M-configuration. Though configurationally stable at room temperature, M-(-)-marinopyrrole A can be racemized at elevated temperatures to yield the non-natural P-(+)-atropo-enantiomer. The marinopyrroles possess potent antibiotic activities against methicillin-resistant Staphylococcus aureus.
Figures
References
-
- Newman DJ, Cragg GM. J Nat Prod. 2007;70:461–477. - PubMed
-
- von Nussbaum F, Brands M, Hinzen B, Weigand S, Haebich D. Angew Chem, Int Ed. 2006;45:5072–5129. - PubMed
-
-
Based on comparisons with DNA–DNA reassociation data, it has been shown that 98% 16S rRNA gene sequence identity is highly conservative in terms of delineating species-level relationships among actinomycetes; see: Stach JEM, Maldonado LA, Masson DG, WARD AC, Goodfellow M, Bull AT. Appl Environ Microbiol. 2003;69:6189–6200.
-
-
- Harada N, Nakanishi K. Circular Dichroic Spectroscopy: Exciton Coupling in Organic Stereochemistry. University Science Books; Mill Valley, CA: 1983.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical