Conformation of human leucocyte antigen-C molecules at the surface of human trophoblast cells
- PMID: 18205788
- PMCID: PMC2440826
- DOI: 10.1111/j.1365-2567.2007.02789.x
Conformation of human leucocyte antigen-C molecules at the surface of human trophoblast cells
Abstract
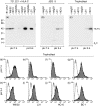
Human leucocyte antigen (HLA)-C is expressed at lower levels than other classical HLA-I molecules on somatic cells. Surface HLA-C proteins can occur as conventionally beta(2)-microglobulin (beta2m)-associated complexes or as open conformers dissociated from peptide and/or beta(2)m. We investigated the conformation of HLA-C molecules on normal human trophoblast cells, which invade the maternal decidua during placentation. A panel of monoclonal antibodies to different conformations of HLA-I molecules was used in flow cytometry and surface immunoprecipitation experiments. On the surface of trophoblast cells only beta(2)m-associated complexes of HLA-C molecules were detected. In contrast, both open conformers and beta(2)m-associated HLA-C could be detected on other cells from the decidua, HLA-C-transfectants and cell lines. The levels of HLA-C expressed on primary trophoblast cells could be detected by antibodies specific to non-beta(2)m-associated conformations because binding was seen after acid-induced denaturation of surface proteins. In contrast to HLA-G molecules on trophoblasts, we found no evidence for the presence of disulphide-linked multimers of HLA-C complexes. These results show that most HLA-C molecules present at the trophoblast cell surface are in the conventional beta(2)m-associated conformation. These findings have implications regarding the stability of trophoblast HLA-C molecules and how they interact with receptors on decidual leucocytes during placentation.
Figures

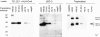
References
-
- Loke YW, King A, Burrows T, et al. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50:135–46. - PubMed
-
- King A, Boocock C, Sharkey AM, Gardner L, Beretta A, Siccardi AG, Loke YW. Evidence for the expression of HLA-C Class I mRNA and protein by human first trimester trophoblast. J Immunol. 1996;156:2068–76. - PubMed
-
- King A, Burrows TD, Hiby SE, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–87. - PubMed
-
- King A, Allan DS, Bowen M, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–31. - PubMed
-
- Verma S, King A, Loke YW. Expression of killer cell inhibitory receptors on human uterine natural killer cells. Eur J Immunol. 1997;27:979–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

