Development of a novel chemokine-mediated in vivo T cell recruitment assay
- PMID: 18206159
- PMCID: PMC2277098
- DOI: 10.1016/j.jim.2007.12.002
Development of a novel chemokine-mediated in vivo T cell recruitment assay
Abstract
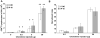
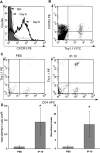
Trafficking of leukocytes to sites of inflammation is an important step in the establishment of an immune response. Chemokines are critical regulators of leukocyte trafficking and are widely studied molecules for their important role in disease and for their potential as new therapeutic targets. The ability of chemokines to induce leukocyte recruitment has been mainly measured by in vitro chemotaxis assays, which lack many components of the complex biological process of leukocyte migration and therefore provide incomplete information about chemokine function in vivo. In vivo assays to study the activity of chemokines to induce leukocyte recruitment have been difficult to establish. We describe here the development of a robust in vivo recruitment assay for CD8(+) and CD4(+) T lymphocytes induced by the CXCR3 ligands IP-10 (CXCL10) and I-TAC (CXCL11). For this assay, in vitro activated T lymphocytes were adoptively transferred into the peritoneum of naïve mice. Homing of these transferred T lymphocytes into the airways was measured following intratracheal instillation of chemokines. High recruitment indices were achieved that were dependent on chemokine concentration and CXCR3 expression on the transferred lymphocytes. Recruitment was also inhibited by antibodies to the chemokine. The assay models the natural condition of chemokine-mediated lymphocyte migration into the airways as chemokines are expressed in the airways during inflammation. The nature of this model allows flexibility to study wildtype and mutant chemokines and chemokine receptors and the ability to evaluate chemokine antagonists and antibodies in vivo. This assay will therefore help elucidate a deeper understanding of the chemokine system in vivo.
Figures








References
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. - PubMed
-
- Campanella GS, Grimm J, Manice LA, Colvin RA, Medoff BD, Wojtkiewicz GR, Weissleder R, Luster AD. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177:6991–8. - PubMed
-
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. - PubMed
-
- Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

