RSV capsid polymorphism correlates with polymerization efficiency and envelope glycoprotein content: implications that nucleation controls morphogenesis
- PMID: 18206161
- PMCID: PMC2268030
- DOI: 10.1016/j.jmb.2007.12.003
RSV capsid polymorphism correlates with polymerization efficiency and envelope glycoprotein content: implications that nucleation controls morphogenesis
Abstract
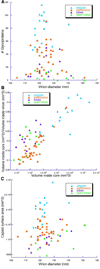
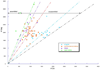
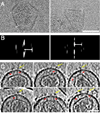
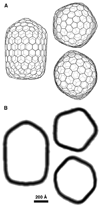
We used cryo-electron tomography to visualize Rous sarcoma virus, the prototypic alpharetrovirus. Its polyprotein Gag assembles into spherical procapsids, concomitant with budding. In maturation, Gag is dissected into its matrix, capsid protein (CA), and nucleocapsid moieties. CA reassembles into cores housing the viral RNA and replication enzymes. Evidence suggests that a correctly formed core is essential for infectivity. The virions in our data set range from approximately 105 to approximately 175 nm in diameter. Their cores are highly polymorphic. We observe angular cores, including some that are distinctively "coffin-shaped" for which we propose a novel fullerene geometry; cores with continuous curvature including, rarely, fullerene cones; and tubular cores. Angular cores are the most voluminous and densely packed; tubes and some curved cores contain less material, suggesting incomplete packaging. From the tomograms, we measured the surface areas of cores and, hence, their contents of CA subunits. From the virion diameters, we estimated their original complements of Gag. We find that Rous sarcoma virus virions, like the human immunodeficiency virus, contain unassembled CA subunits and that the fraction of CA that is assembled correlates with core type; angular cores incorporate approximately 80% of the available subunits, and open-ended tubes, approximately 30%. The number of glycoprotein spikes is variable (approximately 0 to 118) and also correlates with core type; virions with angular cores average 82 spikes, whereas those with tubular cores average 14 spikes. These observations imply that initiation of CA assembly, in which interactions of spike endodomains with the Gag layer play a role, is a critical determinant of core morphology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

