Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1 low mouse model of the disease
- PMID: 18206727
- PMCID: PMC2747096
- DOI: 10.1016/j.exphem.2007.10.001
Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1 low mouse model of the disease
Abstract
Objective: To assess whether alterations in the stromal cell-derived factor-1 (SDF-1)/CXCR4 occur in patients with primary myelofibrosis (PMF) and in Gata1 low mice, an animal model for myelofibrosis, and whether these abnormalities might account for increased stem/progenitor cell trafficking.
Materials and methods: In the mouse, SDF-1 mRNA levels were assayed in liver, spleen, and marrow. SDF-1 protein levels were quantified in plasma and marrow and CXCR4 mRNA and protein levels were evaluated on stem/progenitor cells and megakaryocytes purified from the marrow. SDF-1 protein levels were also evaluated in plasma and in marrow biopsy specimens obtained from normal donors and PMF patients.
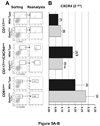
Results: In Gata1 low mice, the plasma SDF-1 protein was five times higher than normal in younger animals. Furthermore, SDF-1 immunostaining of marrow sections progressively increased with age. Similar abnormalities were observed in PMF patients. In fact, plasma SDF-1 levels in PMF patients were significantly higher (by twofold) than normal (p < 0.01) and SDF-1 immunostaining of marrow biopsy specimens demonstrated increased SDF-1 deposition in specific areas. In two of the patients, SDF-1 deposition was normalized by curative therapy with allogenic stem cell transplantation. Similar to what already has been reported for PMF patients, the marrow from Gata1 low mice contained fewer CXCR4 pos CD117 pos cells and these cells expressed low levels of CXCR4 mRNA and protein.
Conclusion: Similar abnormalities in the SDF-1/CXCR4 axis are observed in PMF patients and in the Gata1 low mice model of myelofibrosis. We suggest that these abnormalities contribute to the increased stem/progenitor cell trafficking observed in this mouse model as well as patients with PMF.
Figures









References
-
- Hoffman R, Xu M, Barosi G. Myelofibrosis with myeloid metaplasia. In: Hoffman R, Benz E Jr, Shattil S, Furie B, Cohen HJ, Silberstein L, McGlave P, editors. Hematology: Basic Principles and Practice. 5th Edition. New York: Churchill Livingstone; 2008. In Press.
-
- Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. - PubMed
-
- Barosi G. Myelofibrosis with myeloid metaplasia: diagnostic definition and prognostic classification for clinical studies and treatment guidelines. J Clin Oncol. 1999;17:2954–2970. - PubMed
-
- Guyot B, Murai K, Fujiara Y, et al. Characterization of a megakaryocyte-specific enhancer of the key hemopoietic transcription factor GATA-1. J Biol Chem. 2006;281:13733–13742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

