Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice
- PMID: 18206868
- PMCID: PMC2275901
- DOI: 10.1016/j.ydbio.2007.12.008
Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice
Abstract
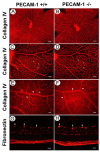
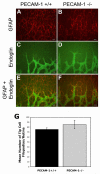
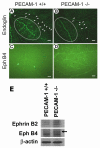
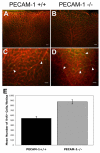
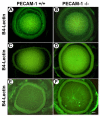
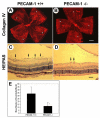
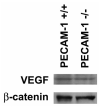
Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) is expressed on the surface of endothelial cells (EC) at high levels with important roles in angiogenesis and inflammation. However, the physiological role PECAM-1 plays during vascular development and angiogenesis remains largely unknown. Here we determined the role of PECAM-1 in the postnatal development of retinal vasculature and retinal neovascularization during oxygen-induced ischemic retinopathy (OIR) using PECAM-1-deficient (PECAM-1-/-) mice. A significant decrease in retinal vascular density was observed in PECAM-1-/- mice compared with PECAM-1+/+ mice. This was attributed to a decreased number of EC in the retinas of PECAM-1-/- mice. An increase in the rate of apoptosis was observed in retinal vessels of PECAM-1-/- mice, which was compensated, in part, by an increase in the rate of proliferation. However, the development and regression of hyaloid vasculature were not affected in the absence of PECAM-1. We did not observe a significant defect in astrocytes, the number of endothelial tip cell filopodias, and the rate of developing retinal vasculature progression in PECAM-1-/- mice. However, we observed aberrant organization of arterioles and venules, decreased secondary branching, and dilated vessels in retinal vasculature of PECAM-1-/- mice. In addition, retinal neovascularization was attenuated in PECAM-1-/- mice during OIR despite an expression of VEGF similar to that of PECAM-1+/+ mice. Mechanistically, these changes were associated with an increase in EphB4 and ephrin B2, and a decrease in eNOS, expression in retinal vasculature of PECAM-1-/- mice. These results suggest that PECAM-1 expression and its potential interactions with EphB4/ephrin B2 and eNOS are important for survival, migration, and functional organization of EC during retinal vascular development and angiogenesis.
Figures




References
-
- Al-Shabrawey M, El-Remessy A, Gu X, Brooks SS, Hamed MS, Huang P, Caldwell RB. Normal vascular development in mice deficient in endothelial NO synthase: possible role of neuronal NO synthase. Mol Vis. 2003;9:549–58. - PubMed
-
- Ando A, Yang A, Mori K, Yamada H, Yamada E, Takahashi K, Saikia J, Kim M, Melia M, Fishman M, Huang P, Campochiaro PA. Nitric oxide is proangiogenic in the retina and choroid. J Cell Physiol. 2002;191:116–24. - PubMed
-
- Bagi Z, Frangos JA, Yeh J-C, White CR, Kaley G, Koller A. PECAM-1 Mediates NO-Dependent Dilation of Arterioles to High Temporal Gradients of Shear Stress. Arterioscler Thromb Vasc Biol. 2005a;25:1590–1595. - PubMed
-
- Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005b;25:1590–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

