Dissection of the osteogenic effects of laminin-332 utilizing specific LG domains: LG3 induces osteogenic differentiation, but not mineralization
- PMID: 18206871
- PMCID: PMC2268764
- DOI: 10.1016/j.yexcr.2007.12.007
Dissection of the osteogenic effects of laminin-332 utilizing specific LG domains: LG3 induces osteogenic differentiation, but not mineralization
Abstract
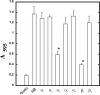
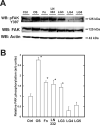
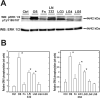
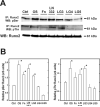
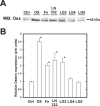
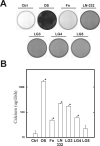
The overall mechanisms governing the role of laminins during osteogenic differentiation of human mesenchymal stem cells (hMSC) are poorly understood. We previously reported that laminin-332 induces an osteogenic phenotype in hMSC and does so through a focal adhesion kinase (FAK) and extracellular signal-related kinase (ERK) dependent pathway. We hypothesized that this is a result of integrin-ECM binding, and that it occurs via the known alpha3 LG3 integrin binding domain of laminin-332. To test this hypothesis we cultured hMSC on several different globular domains of laminin-332. hMSC adhered best to the LG3 domain, and this adhesion maximally activated FAK and ERK within 120 min. Prolonged culturing (8 or 16 days) of hMSC on LG3 led to activation of the osteogenic transcription factor Runx2 and expression of key osteogenic markers (osterix, bone sialoprotein 2, osteocalcin, alkaline phosphatase, extracellular calcium) in hMSC. LG3 domain binding did not increase matrix mineralization, demonstrating that the LG3 domain alone is not sufficient to induce complete osteogenic differentiation in vitro. We conclude that the LG3 domain mediates attachment of hMSC to laminin-332 and that this adhesion recapitulates most, but not all, of the osteogenic differentiation associated with laminin-5 binding to hMSC.
Figures



References
-
- Engvall E. Laminin variants: why, where and when? Kidney Int. 1993;43:2–6. - PubMed
-
- Malinda KM, Kleinman HK. The laminins. Int.J.Biochem.Cell Biol. 1996;28:957–959. - PubMed
-
- Nguyen NM, Senior RM. Laminin isoforms and lung development: all isoforms are not equal. Dev.Biol. 2006;294:271–279. - PubMed
-
- Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J.Cell Sci. 1997;110(Pt 1):55–63. - PubMed
-
- Kingsley K, Huff JL, Rust WL, Carroll K, Martinez AM, Fitchmun M, Plopper GE. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem.Biophys.Res.Commun. 2002;293:1000–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

