Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation
- PMID: 18206970
- PMCID: PMC2327256
- DOI: 10.1016/j.molcel.2007.11.002
Histone H2A monoubiquitination represses transcription by inhibiting RNA polymerase II transcriptional elongation
Abstract
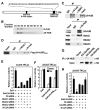
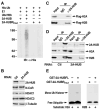
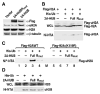
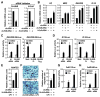
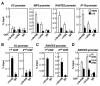
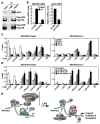
Solving the biological roles of covalent histone modifications, including monoubiquitination of histone H2A, and the molecular mechanisms by which these modifications regulate specific transcriptional programs remains a central question for all eukaryotes. Here we report that the N-CoR/HDAC1/3 complex specifically recruits a specific histone H2A ubiquitin ligase, 2A-HUB/hRUL138, to a subset of regulated gene promoters. 2A-HUB catalyzes monoubiquitination of H2A at lysine 119, functioning as a combinatoric component of the repression machinery required for specific gene regulation programs. Thus, 2A-HUB mediates a selective repression of a specific set of chemokine genes in macrophages, critically modulating migratory responses to TLR activation. H2A monoubiquitination acts to prevent FACT recruitment at the transcriptional promoter region, blocking RNA polymerase II release at the early stage of elongation. We suggest that distinct H2A ubiquitinases, each recruited based on interactions with different corepressor complexes, contribute to distinct transcriptional repression programs.
Figures
References
-
- Amendt BA, Sutherland LB, Semina EV, Russo AF. The molecular basis of Rieger syndrome. Analysis of Pitx2 homeodomain protein activities. The Journal of biological chemistry. 1998;273:20066–20072. - PubMed
-
- Bondarenko VA, Steele LM, Ujvari A, Gaykalova DA, Kulaeva OI, Polikanov YS, Luse DS, Studitsky VM. Nucleosomes can form a polar barrier to transcript elongation by RNA polymerase II. Molecular cell. 2006;24:469–479. - PubMed
-
- Borden KL. RING domains: master builders of molecular scaffolds? J Mol Biol. 2000;295:1103–1112. - PubMed
-
- Breiling A, Turner BM, Bianchi ME, Orlando V. General transcription factors bind promoters repressed by Polycomb group proteins. Nature. 2001;412:651–655. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

