Current and future uses of neuroimaging for cognitively impaired patients
- PMID: 18207114
- PMCID: PMC2728702
- DOI: 10.1016/S1474-4422(08)70019-X
Current and future uses of neuroimaging for cognitively impaired patients
Abstract
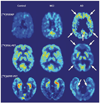
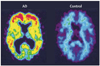
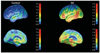
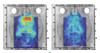
Technological advances have led to greater use of both structural and functional brain imaging to assist with the diagnosis of dementia for the increasing numbers of people with cognitive decline as they age. In current clinical practice, structural imaging (CT or MRI) is used to identify space-occupying lesions and stroke. Functional methods, such as PET scanning of glucose metabolism, could be used to differentiate Alzheimer's disease from frontotemporal dementia, which helps to guide clinicians in symptomatic treatment strategies. New neuroimaging methods that are currently being developed can measure specific neurotransmitter systems, amyloid plaque and tau tangle concentrations, and neuronal integrity and connectivity. Successful co-development of neuroimaging surrogate markers and preventive treatments might eventually lead to so-called brain-check scans for determining risk of cognitive decline, so that physicians can administer disease-modifying medications, vaccines, or other interventions to avoid future cognitive losses and to delay onset of disease.
Conflict of interest statement
The University of California, Los Angeles, owns a US patent (6,274,119) entitled “Methods for labeling β-amyloid plaques and neurofibrillary tangles”, which uses an approach outlined in this Review and has been licensed to Siemens. GWS, GMC, S-CH, and JRB are among the inventors, have received royalties, and will receive royalties on future sales. GWS has served as a consultant and/or received lecture fees from Abbott, Dakim, Eisai, Forest, Myriad Genetics, Novartis, Ortho-McNeil, Pfizer, Radica, Servier, Siemens, and VerusMed. GWS has also received stock options from Dakim. S-CH has received lecture fees from GlaxoSmithKline. JRB has served as a consultant and received lecture fees from Nihon Medi-Physics Co, Bristol-Meyer Squibb, PETNet Pharmaceuticals, and Siemens.
Figures



Similar articles
-
Imaging of neurodegenerative cognitive and behavioral disorders: practical considerations for dementia clinical practice.Handb Clin Neurol. 2016;136:971-84. doi: 10.1016/B978-0-444-53486-6.00050-8. Handb Clin Neurol. 2016. PMID: 27430453 Review.
-
Magnetic resonance imaging and positron emission tomography in the diagnosis of neurodegenerative dementias.Funct Neurol. 2016 Oct/Dec;31(4):205-215. doi: 10.11138/fneur/2016.31.4.205. Funct Neurol. 2016. PMID: 28072381 Free PMC article. Review.
-
Comparison of brief olfactory and cognitive assessments to neuroimaging biomarkers in the prediction of cognitive decline and dementia in the MCSA cohort.Alzheimers Dement. 2024 Dec;20(12):8346-8358. doi: 10.1002/alz.14261. Epub 2024 Oct 10. Alzheimers Dement. 2024. PMID: 39387454 Free PMC article.
-
Clinical impact of (11)C-Pittsburgh compound-B positron emission tomography carried out in addition to magnetic resonance imaging and single-photon emission computed tomography on the diagnosis of Alzheimer's disease in patients with dementia and mild cognitive impairment.Psychiatry Clin Neurosci. 2015 Dec;69(12):741-51. doi: 10.1111/pcn.12326. Epub 2015 Jul 30. Psychiatry Clin Neurosci. 2015. PMID: 26085054
-
Tau PET in autosomal dominant Alzheimer's disease: relationship with cognition, dementia and other biomarkers.Brain. 2019 Apr 1;142(4):1063-1076. doi: 10.1093/brain/awz019. Brain. 2019. PMID: 30753379 Free PMC article.
Cited by
-
Neuroimaging in dementia.Neurotherapeutics. 2011 Jan;8(1):82-92. doi: 10.1007/s13311-010-0012-2. Neurotherapeutics. 2011. PMID: 21274688 Free PMC article. Review.
-
The diagnosis and management of mild cognitive impairment: a clinical review.JAMA. 2014 Dec 17;312(23):2551-61. doi: 10.1001/jama.2014.13806. JAMA. 2014. PMID: 25514304 Free PMC article. Review.
-
Epidemiology of Alzheimer disease.Nat Rev Neurol. 2011 Mar;7(3):137-52. doi: 10.1038/nrneurol.2011.2. Epub 2011 Feb 8. Nat Rev Neurol. 2011. PMID: 21304480 Free PMC article. Review.
-
PET imaging of neural activity, β-amyloid, and tau in normal brain aging.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):3859-3871. doi: 10.1007/s00259-021-05230-5. Epub 2021 Mar 5. Eur J Nucl Med Mol Imaging. 2021. PMID: 33674892 Review.
-
Transcranial Magnetic Stimulation to Address Mild Cognitive Impairment in the Elderly: A Randomized Controlled Study.Behav Neurol. 2015;2015:287843. doi: 10.1155/2015/287843. Epub 2015 Jun 16. Behav Neurol. 2015. PMID: 26160997 Free PMC article. Clinical Trial.
References
-
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. - PubMed
-
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol. 2003;60:1385–1389. - PubMed
-
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. - PubMed
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (IV-Tr) 4th edn—text revised. Washington, DC: American Psychiatric Association; 2000.
-
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease—report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

