Plasma membrane deformation by circular arrays of ESCRT-III protein filaments
- PMID: 18209100
- PMCID: PMC2213594
- DOI: 10.1083/jcb.200707031
Plasma membrane deformation by circular arrays of ESCRT-III protein filaments
Abstract
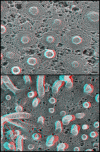
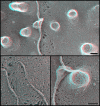
Endosomal sorting complex required for transport III (ESCRT-III) proteins function in multivesicular body biogenesis and viral budding. They are recruited from the cytoplasm to the membrane, where they assemble into large complexes. We used "deep-etch" electron microscopy to examine polymers formed by the ESCRT-III proteins hSnf7-1 (CHMP4A) and hSnf7-2 (CHMP4B). When overexpressed, these proteins target to endosomes and the plasma membrane. Both hSnf7 proteins assemble into regular approximately 5-nm filaments that curve and self-associate to create circular arrays. Binding to a coexpressed adenosine triphosphate hydrolysis-deficient mutant of VPS4B draws these filaments together into tight circular scaffolds that bend the membrane away from the cytoplasm to form buds and tubules protruding from the cell surface. Similar buds develop in the absence of mutant VPS4B when hSnf7-1 is expressed without its regulatory C-terminal domain. We demonstrate that hSnf7 proteins form novel membrane-attached filaments that can promote or stabilize negative curvature and outward budding. We suggest that ESCRT-III polymers delineate and help generate the luminal vesicles of multivesicular bodies.
Figures







References
-
- Babst, M. 2005. A protein's final ESCRT. Traffic. 6:2–9. - PubMed
-
- Babst, M., D.J. Katzmann, E.J. Estepa-Sabal, T. Meerloo, and S.D. Emr. 2002. a. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 3:271–282. - PubMed
-
- Babst, M., D.J. Katzmann, W.B. Snyder, B. Wendland, and S.D. Emr. 2002. b. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 3:283–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

