Skin stem cells: rising to the surface
- PMID: 18209104
- PMCID: PMC2213592
- DOI: 10.1083/jcb.200708185
Skin stem cells: rising to the surface
Abstract
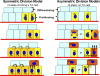
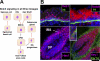
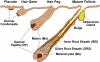
The skin epidermis and its appendages provide a protective barrier that is impermeable to harmful microbes and also prevents dehydration. To perform their functions while being confronted with the physicochemical traumas of the environment, these tissues undergo continual rejuvenation through homeostasis, and, in addition, they must be primed to undergo wound repair in response to injury. The skin's elixir for maintaining tissue homeostasis, regenerating hair, and repairing the epidermis after injury is its stem cells, which reside in the adult hair follicle, sebaceous gland, and epidermis. Stem cells have the remarkable capacity to both self-perpetuate and also give rise to the differentiating cells that constitute one or more tissues. In recent years, scientists have begun to uncover the properties of skin stem cells and unravel the mysteries underlying their remarkable capacity to perform these feats. In this paper, I outline the basic lineages of the skin epithelia and review some of the major findings about mammalian skin epithelial stem cells that have emerged in the past five years.
Figures

Comment in
-
Cell biology of stem cells: an enigma of asymmetry and self-renewal.J Cell Biol. 2008 Jan 28;180(2):257-60. doi: 10.1083/jcb.200712159. J Cell Biol. 2008. PMID: 18227277 Free PMC article.
Similar articles
-
Building epithelial tissues from skin stem cells.Cold Spring Harb Symp Quant Biol. 2008;73:333-50. doi: 10.1101/sqb.2008.73.032. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022769 Free PMC article. Review.
-
miRNAs, 'stemness' and skin.Trends Biochem Sci. 2008 Dec;33(12):583-91. doi: 10.1016/j.tibs.2008.09.002. Epub 2008 Oct 8. Trends Biochem Sci. 2008. PMID: 18848452 Review.
-
Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address.Curr Top Dev Biol. 2016;116:357-74. doi: 10.1016/bs.ctdb.2015.11.033. Epub 2016 Feb 8. Curr Top Dev Biol. 2016. PMID: 26970628 Free PMC article. Review.
-
Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche.Dev Cell. 2017 Nov 20;43(4):387-401. doi: 10.1016/j.devcel.2017.10.001. Dev Cell. 2017. PMID: 29161590 Free PMC article. Review.
-
More than one way to skin . .Genes Dev. 2008 Apr 15;22(8):976-85. doi: 10.1101/gad.1645908. Genes Dev. 2008. PMID: 18413712 Free PMC article. Review.
Cited by
-
Stem cell expansion during carcinogenesis in stem cell-depleted conditional telomeric repeat factor 2 null mutant mice.Oncogene. 2013 Oct 24;32(43):5156-66. doi: 10.1038/onc.2012.555. Epub 2012 Nov 26. Oncogene. 2013. PMID: 23178498 Free PMC article.
-
miR-136 modulates TGF-β1-induced proliferation arrest by targeting PPP2R2A in keratinocytes.Biomed Res Int. 2015;2015:453518. doi: 10.1155/2015/453518. Epub 2015 Jan 14. Biomed Res Int. 2015. PMID: 25654102 Free PMC article.
-
Loss-of-function FERMT1 mutations in kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation.Am J Pathol. 2009 Oct;175(4):1431-41. doi: 10.2353/ajpath.2009.081154. Epub 2009 Sep 17. Am J Pathol. 2009. PMID: 19762710 Free PMC article.
-
LGR5 is a conserved marker of hair follicle stem cells in multiple species and is present early and throughout follicle morphogenesis.Sci Rep. 2022 Jun 1;12(1):9104. doi: 10.1038/s41598-022-13056-w. Sci Rep. 2022. PMID: 35650234 Free PMC article.
-
Overabundance of putative cancer stem cells in human skin keratinocyte cells malignantly transformed by arsenic.Toxicol Sci. 2012 Jan;125(1):20-9. doi: 10.1093/toxsci/kfr282. Epub 2011 Oct 19. Toxicol Sci. 2012. PMID: 22011395 Free PMC article.
References
-
- Andl, T., S.T. Reddy, T. Gaddapara, and S.E. Millar. 2002. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2:643–653. - PubMed
-
- Andl, T., K. Ahn, A. Kairo, E.Y. Chu, L. Wine-Lee, S.T. Reddy, N.J. Croft, J.A. Cebra-Thomas, D. Metzger, P. Chambon, et al. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 131:2257–2268. - PubMed
-
- Arnold, I., and F.M. Watt. 2001. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11:558–568. - PubMed
-
- Blanpain, C., W.E. Lowry, A. Geoghegan, L. Polak, and E. Fuchs. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 118:635–648. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

